Abstract
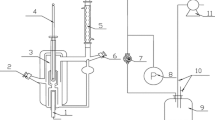
Isothermal vapor–liquid equilibrium data have been measured for the binary system R32 (difluoromethane) + SO2 at eight temperatures between 288.07 and 403.16 K, and at pressures in the range 0.417–7.31 MPa. The experimental method used in this work is of the static–analytic type, taking advantage of two pneumatic capillary samplers (RolsiTM, Armines’ patent) developed in the CENERG/TEP laboratory. The data were measured with uncertainties within ±0.02 K and ±0.0015 MPa, respectively, for temperatures and pressures and ±1% for molar compositions as a result of careful calibrations. The isothermal P, x, y data are well represented with the Peng–Robinson equation of state using the Mathias–Copeman alpha function and the Wong–Sandler mixing rules involving the NRTL model.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valtz, A., Coquelet, C. & Richon, D. Vapor–Liquid Equilibrium Data for the Sulfur Dioxide (SO2) + Difluoromethane (R32) System at Temperatures from 288.07 to 403.16 K and at Pressures up to 7.31 MPa. Int J Thermophys 25, 1695–1711 (2004). https://doi.org/10.1007/s10765-004-7730-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10765-004-7730-9