Abstract
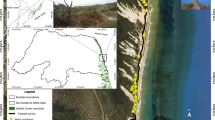
Studies of primate foraging efficiency during the exploration of new areas can provide important insights into the adaptive value of long-term spatial memory. After 6 yr of observation of a group of gray-cheeked mangabeys (Lophocebus albigena johnstonii) in Kibale National Park, Uganda, we observed exploration of a new area, followed 7 mo later by a group split. We recorded their ranging and foraging behavior for 22 mo after the first exploration. Controlling for weather variables, we found that mangabeys moved longer daily travel distances, explored more area per day, and had larger group spreads in the new area compared to the old area in both parent and daughter groups. The increase in search swath in the new area likely enabled the monkeys to counteract their lack of knowledge of food locations in the new area, as the efficiency in finding fruit in general did not differ between the old and new areas. We did, however, find a lower efficiency in finding fruit from preferred fig trees whose edibility could not be assessed by visual cues in the new area. Fig finding efficiency remained lower, even when we controlled for potential differences in fig density. In addition, mangabeys traveled and foraged less often on the ground in the new compared to the old area. However, when the monkeys became more familiar with the new area, terrestrial behavior increased. Our results are consistent with the hypothesis that when monkeys move into an area in which they have no experience, an absence of knowledge acquired via long-term spatial memory decreases their foraging efficiency.





Similar content being viewed by others
References
Altmann, S. A., & Altmann, J. (1970). Baboon ecology: African field research. Basel: Karger.
Aronsen, G. P. (2004). Locomotor energetics and forest canopy variation: implications for conservation and management. Folia Primatologica Supplement, 1, 233.
Balda, R. P. & Kamil, A. C. (1998). The ecology and evolution of spatial memory in corvids of the Southwestern USA: The perplexing pinyon jay. In R. O. Balda, I. M. Pepperberg & A. C. Kamil (Eds.), Animal cognition in nature: The convergence of psychology and biology in laboratory and field (pp. 29–64). San Diego, CA: Academic Press.
Barrett, L. (1995). Foraging strategies, ranging patterns and territoriality among gray-cheeked mangabeys in Kibale forest, Western Uganda. Ph.D. thesis, University College London.
Bronikowski, M., & Altmann, J. (1996). Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology, 39, 11–25.
Brown, C. (2001). Familiarity with the test environment improves escape responses in the crimson spotted rainbowfish, Melanotaenia duboulayi. Animal Cognition, 4, 109–113.
Chalmers, N. R. (1968). Group composition, ecology and daily activities of free living mangabeys in Uganda. Folia Primatologica, 8, 247–262.
Chapman, C. A., Chapman, L. J., Wrangham, R., Isabirye-Basuta, G., & Ben-David, K. (1997). Spatial and temporal variability in the structure of a tropical forest. African Journal of Ecology, 35, 341–436.
Chapman, C. A., Wrangham, R. W., Chapman, L. J., Kennard, D. K., & Zanne, A. E. (1999). Fruit and flower phenology at two sites in Kibale National Park, Uganda. Journal of Tropical Ecology, 15, 189–211.
Chepko-Sade, B. D., & Sade, D. S. (1979). Patterns of group splitting within matrilineal kinship groups: a study of social group structure in Macaca mulatta (Cercopithecine: Primates). Behavioral Ecology and Sociobiology, 5, 67–87.
Cords, M., & Rowell, T. E. (1986). Group fission in blue monkeys of the Kakamega Forest, Kenya. Folia Primatologica, 46, 70–82.
Crook, J. H. (1965). The adaptive significance of avian social organization. Symposia of the Zoological Society of London, 14, 181–218.
Di Fiore, A., & Suarez, S. A. (2007). Route-based travel and shared routes in sympatric spider and woolly monkeys: cognitive and evolutionary implications. Animal Cognition, 10, 317–329.
Doolan, S. P., & MacDonald, D. W. (1996). Dispersal and extra-territorial prospecting by slender tailed meerkats (Suricata suricatta) in the south-western Kalahari. Journal of Zoology, 240, 59–73.
Emery, N. J., & Clayton, N. S. (2004). Comparing the complex cognition of birds and primates. In L. J. Rogers & G. Kaplan (Eds.), Comparative vertebrate cognition (pp. 3–55). The Hague: Kluwer Academic Press.
Forsman, J. T., Mönkkönen, M., Inkeröinen, J., & Reunanen, P. (1998). Aggregate dispersion of birds after encountering a predator: experimental evidence. Journal of Avian Biology, 29, 44–48.
Garber, P. A. (1989). Role of spatial memory in primate foraging patterns Saguinus mystax and Saguinus fuscicollis. American Journal of Primatology, 19, 203–216.
Girvan, J. R. & Braithwaite, V. A. (1997). Orientation mechanisms in different populations of the three spined stickleback. In Orientation and Navigation – birds, humans and other animals. Oxford: Royal institute of Navigation.
Hamilton, W. D. (1971). Geometry for the selfish herd. Journal of Theoretical Biology, 31, 295–311.
Isbell, L. A., & van Vuren, D. (1996). Differential cost of locational and social dispersal and their consequences for female group-living primates. Behaviour, 133, 1–36.
Isbell, L. A., Cheney, D. L., & Seyfarth, R. M. (1990). Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behavioral Ecology and Sociobiology, 27, 351–358.
Janmaat, K. R. L. (2006). Fruits of enlightenment: Fruit finding strategies in wild mangabey monkeys. Ph.D. thesis, University of St. Andrews.
Janmaat, K. R. L., Byrne, R. W., & Zuberbühler, K. (2006a). Evidence for spatial memory of fruiting states of rain forest fruit in wild ranging mangabeys. Animal Behaviour, 71, 797–807.
Janmaat, K. R. L., Byrne, R. W., & Zuberbühler, K. (2006b). Primates take weather into account when searching for fruit. Current Biology, 16, 1232–1237.
Janmaat, K. R. L., Olupot, W., Chancellor, R. L., Arlet, M. E., & Waser, P. M. (2009). Long-term site fidelity and individual home range shifts in Lophocebus albigena. International Journal of Primatology, 30, 443–466.
Janmaat, K. R. L., Mijer, R., Chapman, C. A., & Zuberbuhler, K. (submitted). The use of fruiting synchrony in foraging mangabeys. Animal Cognition
Janson, C. H. (1998). Experimental evidence for spatial memory in foraging wild capuchin monkeys Cebus apella. Animal Behaviour, 55, 1229–1243.
Janson, C. H., & Di Bitetti, M. S. (1997). Experimental analysis of food detection in capuchin monkeys: effects of distance, travel speed, and resource size. Behavioral Ecology and Sociobiology, 41, 17–24.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of rhesus macaques (Macaca mulatta). American Journal of Primatology, 44, 71–82.
Krebs, C. J. (1988). Population ecology of individuals (pp. 1059–1060). Princeton: Princeton University Press.
Larsen, K. W., & Boutin, S. (1994). Movements, survival and settlement of red squirrel (Tamiasciurus hudsonicus) offspring. Ecology, 70, 214–223.
Manzer, M. B., & Bell, M. B. (2004). Spatial representation of shelter locations in meerkats, Suricata suricatta. Animal Behaviour, 68, 151–157.
Martin, P., & Bateson, P. (1986). Measuring behavior. Cambridge, UK: Cambridge University Press.
Menzel, E. W. (1973). Chimpanzee spatial memory organization. Science, 182, 943–945.
Mermier, C. M., Robergs, R. A., McMinn, S. M., & Heyward, V. H. (1997). Energy expenditure and physiological responses during indoor rock climbing. British Journal of Sports Medicine, 31, 224–228.
Metzgar, L. H. (1967). An experimental comparison of screech owl predation on resident and transient white-footed mice Peromyscus leucopus. Journal of Mammalogy, 48, 387–391.
Milton, K. (1988). Foraging behaviour and the evolution of primate intelligence. In R.W. Byrne and A. Whiten (Eds.), Machiavellian Intelligence: Social expertise and the evolution of intellect in monkeys, apes and humans (pp. 285–305). Oxford: Clarendon Press.
Murray, C. M., Gilby, I. C., Mane, S. V., & Pusey, A. E. (2008). Adult male chimpanzees inherit maternal ranging patterns. Current Biology, 18, 20–24.
Muruthi, P., Altmann, J., & Altmann, S. (1991). Resource base, parity, and reproductive condition affect females’ feeding time and nutrient intake within and between groups of a baboon population. Oecologia, 87, 467–472.
Newton-Fisher, N. E. (2003). The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. African Journal of Ecology, 41, 150–156.
Nilsson, J. A. (1989). Causes and consequences of natal dispersal in marsh tit, Parus palustris. Journal of Animal Ecology, 58, 619–636.
Oates, J. F. (1978). Water-plant and soil consumption by guereza monkeys (Colobus guereza): a relationship with minerals and toxins in the diet? Biotropica, 10, 241–253.
Olupot, W. (1998). Long-term variation in mangabey (Cercocebus albigena johnstoni Lydekker) feeding in Kibale National Park, Uganda. African Journal of Ecology, 36, 96–101.
Olupot, W. (1999). Mangabey dispersal and conservation in Kibale National Park, Uganda. Ph.D. thesis, Purdue University.
Olupot, W. (2000). Body mass differences among male mangabeys inhabiting logged and unlogged forest compartments. Conservation Biology, 14, 833–843.
Perneger, T. V. (1998). What’s wrong with Bonferroni adjustments? British Medical Journal, 316, 1236–1238.
Poirier, F. E. (1968). Analysis of a Nilgiri langur (Presbytis johnii) home range change. Primates, 9, 29–43.
Poulsen, J. R., & Clark, C. J. (2007). Predation on mammals by the grey-cheeked mangabey Lophocebus albigena. Primates, 42, 391–394.
Reed, J. M., Boulinier, T., Danchin, E., & Oring, L. W. (1999). Prospecting by birds for breeding sites. Current Ornithology, 15, 189–259.
Roper, T. J., Ostler, J. R., & Conradt, L. (2003). The process of dispersal in badgers Meles meles. Mammal Review, 33, 314–318.
Scheumann, M., & Call, J. (2006). Sumatran orangutans and a yellow-cheeked crested gibbon know what is where. International Journal of Primatology, 27, 575–601.
Shultz, S., Faurie, C., & Noe, R. (2003). Behavioral responses of Diana monkeys to male long-distance calls: changes in ranging, association patterns and activity. Behavioral Ecology and Sociobiology, 53, 238–245.
Singleton, I., & van Schaik, C. P. (2001). Orangutan home range size and its determinants in a Sumatran swamp forest. International Journal of Primatology, 22, 877–911.
Sokal, R. R., & Rohlf, F. J. (1981). Biometry: The principles and practice of statistics in biological research. San Francisco: W. H. Freeman.
Steudel, K. (2000). The physiology and energetics of movement effects on individual and groups. In S. Boinski & P. A. Garber (Eds.), On the move: How and why animals travel in groups (pp. 9–23). Chicago: The University of Chicago Press.
Stevens, J., Rosati, A., Ross, K., & Hauser, M. (2005). Will travel for food: spatial discounting in two new world monkeys. Current Biology, 15, 1855–1860.
Struhsaker, T. T. (1997). Ecology of an African rainforest. Gainseville, FL: University Press of Florida.
Struhsaker, T. T., & Leakey, M. (1990). Prey selectivity by crowned hawk-eagles on monkeys in Kibale Forest, Uganda. Behavioral Ecology and Sociobiology, 26, 435–443.
Struhsaker, T. T., & Leland, L. (1988). Group fission in redtail monkeys (Cercopithecus ascanius) in the Kibale forest, Uganda. In A. Gautier-Hion, F. Bourlière, J. Gautier, & J. Kingdon (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 364–388). New York: Cambridge University Press.
Sugiyama, Y. (1960). On the division of a natural troop of Japanese monkeys at Takasakiyama. Primates, 2, 109–148.
Tolman, E. C. (1948). Cognitive maps in rats and men. The Psychological Review 55, 189–208.
Tinbergen, N. (1972). The animal in its world. Cambridge: Harvard Press.
Wallis, S. J. (1979). The socioecology of Cercocebus albigena johnstonii (Lyddeker): An arboreal rainforest monkey. Ph.D. thesis, University of London.
Waser, P. M. (1974). Intergroup interactions in a forest monkey: The mangabey Cercocebus albigena. Ph.D. thesis, the Rockefeller University.
Waser, P. M. (1976). Cercocebus albigena: site attachment, avoidance, and intergroup spacing. American Naturalist, 11, 91–933.
Waser, P. M. (1977). Individual recognition, intragroup cohesion, and intergroup spacing: evidence for sound playback to forest monkeys. Behaviour, 60, 28–74.
Waser, P. M. (1985). Spatial structure in mangabey groups. International Journal of Primatology, 6, 569–580.
Waser, P. M., & Floody, O. (1974). Ranging patterns of the mangabey Cercocebus albigena, in the Kibale Forest, Uganda. Zeitschrift fur Tierpsychologie, 35, 2–101.
Waser, P. M., & Jones, W. T. (1983). Natal philopatry among solitary mammals. Quarterly Review of Biology, 58, 355–390.
Windberg, L. A. (1996). Coyote responses to visual and olfactory stimuli related to familiarity with an area. Canadian Journal of Zoology, 74, 2248–2253.
Zuberbühler K, Janmaat KRL (2010). Foraging cognition in non-human primates. In: Platt ML & Ghazanfar AA (Eds.), Primate Neuroethology (pp. 64–83). Oxford: Oxford University Press.
Acknowledgments
The Wenner-Gren and Leakey Foundation, the University of St. Andrews’ School of Psychology, the Schure-Bijerinck-Popping Foundation of the KNAW, the Stichting Kronendak, the Dobberke Stichting voor Vergelijkende Psychology, the Lucie Burger Stichting, and the Foundation Doctor Catharine van Tussenbroek provided funding to K. R. L. Janmaat. The Leakey Foundation and the University of California, Davis Department of Anthropology provided funding to R. L. Chancellor. We thank the Office of the President, the Uganda National Council for Science and Technology, the Uganda Wildlife Authority, the Makerere University Biological Field Station, and the Kibale Fish and Monkey Project, C. A. Chapman in particular, for logistic support, permission to conduct research in Kibale National Park, and the rainfall and temperature data needed to conduct our analyses. We thank W. Olupot and G. Arlet for sending us their ranging data and for training our assistants. We thank R. Meijer, L. B. Prevot, D. C. M. Wright, J. Rusoke, P. Irumba, R. Kaserengenyu, S. Katusabe, and R. Sabiiti for invaluable assistance in the field. We thank L. A. Isbell, K. Zuberbühler, C. H. Janson, G. Brown, 3 anonymous reviewers, and P. M. Waser in particular for comments and suggestions that significantly improved earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 3555 kb)
Rights and permissions
About this article
Cite this article
Janmaat, K.R.L., Chancellor, R.L. Exploring New Areas: How Important is Long-Term Spatial Memory for Mangabey (Lophocebus albigena johnstonii) Foraging Efficiency?. Int J Primatol 31, 863–886 (2010). https://doi.org/10.1007/s10764-010-9433-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9433-3