Abstract
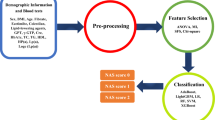
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic disease worldwide, consisting of a broad spectrum of diseases such as simple steatosis (NAFL), non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma. Hepatic inflammation plays a key role in the pathophysiology of NAFLD. Inflammatory mediators such as cytokines and chemokines are considered as contributing factors to NAFLD development and progression. In the present study, we aimed to investigate the inflammatory protein signatures as predictive disease-specific markers for non-alcoholic fatty liver disease (NAFLD). This cross-sectional study included healthy control (n = 64), NAFL (n = 109), and NASH (n = 60) human subjects. Serum concentrations of various cytokines and chemokines were evaluated using sensitive multiplex assays. We used principal component analysis (PCoA) to reveal distinct differences in the levels of cytokines and chemokines between each of the study groups. Further, a random forest classification model was developed to identify the panel of markers that could predict diseases. The protein–protein network analysis was performed to determine the various signaling pathways associated with the disease-specific panel of markers. Serum concentrations of TNF-α, IL-1β, IL-1ra, G-CSF, PDGF-BB, MCP-1, MIP-1a, MIP-1b, RANTES, eotaxin, IL-8 and IP-10 were significantly increased in NASH group as compared to control group. Furthermore, serum concentrations of IL-9 and IL-13 were significantly lower in the NASH group, whereas IL-2 levels were significantly decreased in the NAFL group when compared to the control group. PCoA results demonstrated statistically significant differences in cytokines and chemokines between each of the study groups (PERMANOVA p = 0.001; R2 = 0.102). RANTES, IL-1ra, MIP-1b, IL-2, and G-CSF could differentiate the NAFL group from the controls; G-CSF, IL-1ra, TNF-α, RANTES, and IL-9 could differentiate the NASH group from the controls; and G-CSF, IL-9, IL-13, eotaxin, and TNF- α could differentiate the NASH group from the NAFL group. Our protein–protein network revealed that these markers are involved in cytokine-cytokine receptor interaction, Th1 and Th2 cell differentiation, TNF, chemokine, JAK/STAT, P13K/Akt, TLR, NOD-like receptor, NF-kB, and adipocytokine signaling pathways which might be responsible for disease pathogenesis. Our study findings revealed a set of distinct cytokine and chemokine markers and they might be considered as biomarkers in distinguishing NASH from NAFL. Future multicentre studies with larger sample size are recommended to determine the potential utility of these panels of markers.





Similar content being viewed by others
Data Availability
Data related to the present study were included in the article and submitted in the supplementary information.
References
Younossi, Z., Q.M. Anstee, M. Marietti, et al. 2018. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nature Reviews. Gastroenterology & Hepatology 15 (1): 11–20.
Estes, C., H. Razavi, R. Loomba, Z. Younossi, A.J. Sanyal, et al. 2018. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in the burden of disease: Estes, et al. Hepatology 67 (1): 123–133.
Francisco, V., M.J. Sanz, J.T. Real, et al. 2022. Adipokines in non-alcoholic fatty liver disease: are we on the road toward new biomarkers and therapeutic targets? Biology 11 (8): 1237.
Kleiner, D.E., E.M. Brunt, M. Van Natta, et al. 2005. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 41 (6): 1313–1321.
Poynard, T., V. Ratziu, S. Naveau, et al. 2005. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comparative Hepatology 4: 10.
Lee, J.-H., D. Kim, H.J. Kim, et al. 2010. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Digestive and Liver Disease 42 (7): 503–508.
Fedchuk, L., F. Nascimbeni, R. Pais, F. Charlotte, C. Housset, and V. Ratziu. 2014. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics 40 (10): 1209–1222.
Sterling, R.K., E. Lissen, N. Clumeck, et al. 2006. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43 (6): 1317–1325.
Kim, D., W.R. Kim, H.J. Kim, and T.M. Therneau. 2013. Association between non-invasive fibrosis markers and mortality among adults with non-alcoholic fatty liver disease in the United States. Hepatology 57 (4): 1357–1365.
Wai, C.-T., J.K. Greenson, R.J. Fontana, et al. 2003. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38 (2): 518–526.
Byrne, C.D., and G. Targher. 2015. NAFLD: A multisystem disease. Journal of Hepatology 62: S47–S64.
Foroughi, M., Z. Maghsoudi, S. Khayyatzadeh, R. Ghiasvand, G. Askari, and B. Iraj. 2016. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Advanced Biomedical Research 5: 28.
Stojsavljevic, S., M. Gomercic Palcic, L. Virovic Jukic, L. Smircic Duvnjak, and M. Duvnjak. 2014. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World Journal of Gastroenterology 20 (48): 18070–18091.
Fricker, Z.P., A. Pedley, J.M. Massaro, et al. 2019. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the framingham heart study. Clinical Gastroenterology and Hepatology 17 (6): 1157-1164.e4.
Auguet, T., L. Bertran, J. Binetti, et al. 2020. Relationship between IL-8 circulating levels and TLR2 hepatic expression in women with morbid obesity and nonalcoholic steatohepatitis. International Journal of Molecular Sciences 21 (11): 4189.
Darmadi, D., and R.H. Ruslie. 2021. Association between serum interleukin (IL)-12 level and severity of non-alcoholic fatty liver disease (NAFLD). Romanian Journal of Internal Medicine 59 (1): 66–72.
Flisiak-Jackiewicz, M., A. Bobrus-Chociej, E. Tarasów, M. Wojtkowska, I. Białokoz-Kalinowska, and D.M. Lebensztejn. 2018. Predictive role of interleukin-18 in liver steatosis in obese children. Canadian Journal of Gastroenterology & Hepatology 2018: 3870454.
Shoji, H., S. Yoshio, Y. Mano, et al. 2016. Interleukin-34 as a fibroblast-derived marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Science and Reports 6: 28814.
Rabelo, F., C.P.M.S. Oliveira, J. Faintuch, et al. 2010. Pro-and Anti-Inflammatory Cytokines in Steatosis and Steatohepatitis. Obesity Surgery 20 (7): 906–912.
Niederreiter, L., and H. Tilg. 2018. Cytokines and Fatty Liver Diseases. Liver Research 2 (1): 14–20.
Majeed, N.A.A., K.S. Ramadan, and O.A. Khalil. 2012. Level of pro-and anti-inflammatory cytokines in non-alcoholic fatty liver disease in Egyptian patients. IRACST International Journal of Research in Management & Technology 2 (2): 2249–9563.
Borroni, G., R. Ceriani, M. Cazzaniga, et al. 2006. Comparison of simple tests for the non-invasive diagnosis of clinically silent cirrhosis in chronic hepatitis C. Alimentary Pharmacology & Therapeutics 24: 797–804.
Angulo, P., J.M. Hui, G. Marchesini, et al. 2007. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45: 846–854.
Zeng, Y., H. He, and Z. An. 2022. Advance of Serum Biomarkers and Combined Diagnostic Panels in Nonalcoholic Fatty Liver Disease. Disease Markers 2022: 1254014.
Fan, J.G., T. Saibara, S. Chitturi, B.I. Kim, J.J. Sung, and A. Chutaputti. 2007. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? Journal of Gastroenterology and Hepatology 22: 794–800.
Hadizadeh, F., E. Faghihimani, and P. Adibi. 2017. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World Journal of Gastrointestinal Pathophysiology 8 (2): 11–26.
Díez-Vallejo, J., and A. Comas-Fuentes. 2011. Asymptomatic hypertransaminasemia in patients in primary care. Revista Espanola de Enfermedades Digestivas 103: 530–535.
Charatcharoenwitthaya, P., K.D. Lindor, and P. Angulo. 2012. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Digestive Diseases and Sciences 57: 1925–1931.
Mahaling, D.U., M.M. Basavaraj, and A.J. Bika. 2013. Comparison of lipid profile in different grades of non-alcoholic fatty liver disease diagnosed on ultrasound. Asian Pacific Journal of Tropical Biomedicine 3 (11): 907–912.
Camell, C., E. Goldberg, and V.D. Dixit. 2015. Regulation of Nlrp3 inflammasome by dietary metabolites. Seminars in Immunology 27 (5): 334–342.
Marra, F., and F. Tacke. 2014. Roles for chemokines in liver disease. Gastroenterology 147 (3): 577–594.
Luci, C., M. Bourinet, P.S. Leclère, R. Anty, and P. Gual. 2020. Chronic inflammation in non-alcoholic steatohepatitis: molecular mechanisms and therapeutic strategies. Frontiers in Endocrinology (Lausanne) 11.
Schwabe, R.F., and D.A. Brenner. 2006. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. American Journal of Physiology - Gastrointestinal Liver Physiology 290 (4): 583–9.
Day, C.P. 2006. From fat to inflammation. Gastroenterology 130: 207–210.
Wieckowska, A., B.G. Papouchado, Z. Li, R. Lopez, N.N. Zein, and A.E. Feldstein. 2008. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. American Journal of Gastroenterology 103: 1372–1379.
Hui, J.M., A. Hodge, G.C. Farrell, J.G. Kench, A. Kriketos, and J. George. 2004. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology 40: 46–54.
Jarrar, M.H., A. Baranova, R. Collantes, et al. 2008. Adipokines and cytokines in non-alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics 27 (5): 412–421.
Baranova, A., K. Schlauch, H. Elariny, et al. 2007. Gene expression patterns in hepatic tissue and visceral adipose tissue of patients with non-alcoholic fatty liver disease. Obesity Surgery 17 (8): 1111–1118.
Loman, B.R., D. Hernández-Saavedra, R. An, and R.S. Rector. 2018. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutrition Reviews 76 (11): 822–839.
Miura, K., L. Yang, N. van Rooijen, D.A. Brenner, H. Ohnishi, and E. Seki. 2013. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 57: 577–589.
Kumar, R., S. Prakash, S. Chhabra, et al. 2012. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian Journal of Medical Research 136: 229–236.
Stienstra, R., F. Saudale, C. Duval, et al. 2010. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology 51: 511–522.
Stanton, M.C., S.C. Chen, J.V. Jackson, et al. 2011. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. Journal of Inflammation (London) 8: 8.
Mollica, M.P., L. Lionetti, R. Putti, G. Cavaliere, M. Gaita, and A. Barletta. 2011. From chronic overfeeding to hepatic injury: Role of endoplasmic reticulum stress and inflammation. Nutrition, Metabolism, and Cardiovascular Diseases 21: 222–230.
Isoda, K., S. Sawada, M. Ayaori, et al. 2005. Deficiency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. Journal of Biological Chemistry 280 (8): 7002–7009.
Perito, E.R., V. Ajmera, N.M. Bass, et al. 2017. Association between cytokines and liver histology in children with nonalcoholic fatty liver disease. Hepatology Communications 1 (7): 609–622.
Du Plessis, J., J. van Pelt, H. Korf, et al. 2015. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 149: 635-648.e14.
Nam, H.H., D.W. Jun, K. Jang, W.K. Saeed, J.S. Lee, H.T. Kang, and Y.J. Chae. 2017. Granulocyte colony-stimulating factor treatment in non-alcoholic fatty liver disease: Beyond marrow cell mobilization. Oncotarget 8 (58): 97965–97976.
Zhang, Y., X. Zhou, P. Liu, et al. 2021. GCSF deficiency attenuates nonalcoholic fatty liver disease through regulating GCSFR-SOCS3-JAK-STAT3 pathway and immune cells infiltration. American Journal of Physiology. Gastrointestinal and Liver Physiology 320 (4): G531–G542.
Harada, M., Y. Qin, H. Takano, et al. 2005. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nature Medicine 11: 305–311.
Kocabayoglu, P., A. Lade, Y.A. Lee, et al. 2015. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. Journal of Hepatology 63: 141–147.
Czochra, P., B. Klopcic, E. Meyer, et al. 2006. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. Journal of Hepatology 45: 419–428.
Kurys-Denis, E., A. Prystupa, D. Luchowska-Kocot, W. Krupski, H. Bis-Wencel, and L. Panasiuk. 2020. PDGF-BB homodimer serum level - a good indicator of the severity of alcoholic liver cirrhosis. Annals of Agricultural and Environmental Medicine 27: 80–85.
Kirchmeyer, M., A. Gaigneaux, F.A. Servais, et al. 2023. Altered profiles of circulating cytokines in chronic liver diseases (NAFLD/HCC): Impact of the PNPLA3I148M risk allele. Hepatol Commun 7 (12): e0306.
Zhou, J., Y. Deng, L. Yan, H. Zhao, and G. Wang. 2016. Serum platelet-derived growth factor-BB levels: A potential biomarker for the assessment of liver fibrosis in patients with chronic hepatitis B. International Journal of Infectious Diseases 49: 94–99.
Chakraborty, S., K.F. Kubatzky, and D.K. Mitra. 2019. An update on interleukin-9: From its cellular source and signal transduction to its role in immunopathogenesis. International Journal of Molecular Sciences 20: 2113.
Varshney, P., R. Parveen, M.A. Khan, S. Kohli, and N.B. Agarwal. 2020. Increased serum interleukin-9 and interleukin-1β are associated with depression in type 2 diabetes patients. Arquivos de Neuro-Psiquiatria 78: 255–261.
Vasanthakumar, R., V. Mohan, G. Anand, M. Deepa, S. Babu, and V. Aravindhan. 2015. Serum IL-9, IL-17, and TGF-β levels in subjects with diabetic kidney disease (CURES-134). Cytokine 72: 109–112.
Shimamura, T., T. Fujisawa, S.R. Husain, M. Kioi, A. Nakajima, and R.K. Puri. 2008. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. The Journal of Immunology 181: 4656–4665.
Weng, H.L., Y. Liu, J.L. Chen, et al. 2009. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology 50: 230–243.
Darkhal, P., M. Gao, Y. Ma, and D. Liu. 2015. Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. International Journal of Obesity 39: 1292–1299.
Duan, Y., X. Pan, J. Luo, X. Xiao, J. Li, P.L. Bestman, and M. Luo. 2022. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Frontiers in Immunology 13.
Haukeland, J.W., J.K. Damås, Z. Konopski, et al. 2006. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. Journal of Hepatology 44: 1167–1174.
Weisberg, S.P., D. Hunter, R. Huber, et al. 2006. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of Clinical Investigation 116: 115–124.
Kirovski, G., E. Gäbele, C. Dorn, et al. 2010. Hepatic steatosis causes induction of the chemokine RANTES in the absence of significant hepatic inflammation. International Journal of Clinical and Experimental Pathology 3 (7): 675–680.
Ponziani, F.R., S. Bhoori, C. Castelli, et al. 2019. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 69: 107–120.
Trifilo, M.J., C.C. Bergmann, W.A. Kuziel, and T.E. Lane. 2003. CC chemokine ligand 3 (CCL3) regulates CD8(+)-T-cell effector function and migration following viral infection. Journal of Virology 77: 4004–4014.
Du Plessis, J., H. Korf, J. Van Pelt, et al. 2016. Pro-Inflammatory cytokines but not endotoxin-related parameters associate with disease severity in patients with NAFLD. PLoS ONE 11.
Pan, X., A.C. Kaminga, A. Liu, S.W. Wen, J. Chen, and J. Luo. 2020. Chemokines in non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Frontiers in Immunology 11: 1802.
Xu, L., Y. Chen, M. Nagashimada, et al. 2021. CC chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice. Metabolism 125: 154914.
Ajmera, V., E.R. Perito, N.M. Bass, et al. 2017. NASH Clinical Research Network. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology 65 (1): 65–77.
Clement, S., S. Pascarella, S. Conzelmann, C. Gonelle-Gispert, K. Guilloux, and F. Negro. 2010. The hepatitis C virus core protein indirectly induces alpha-smooth muscle actin expression in hepatic stellate cells via interleukin-8. Journal of Hepatology 52: 635–643.
Ullah, A., A. Ud Din, W. Ding, et al. 2023. A narrative review: CXC chemokines influence immune surveillance in obesity and obesity-related diseases: Type 2 diabetes and nonalcoholic fatty liver disease. Reviews in Endocrine & Metabolic Disorders 24: 611–631.
Xu, Z., X. Zhang, J. Lau, and J. Yu. 2016. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: Role as a pro-inflammatory factor and clinical implication. Expert Reviews in Molecular Medicine 18: e16.
Acknowledgements
We gratefully acknowledge the support from the Director, NIPER-Guwahati and the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India. The authors thank Dr. Rinku and Dr.Dharitree for providing access and support while operating the Bio-plex device at GMCH, Guwahati.
Funding
This research study was funded by the Institutional Core Grant, NIPER-Guwahati, Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
NM, UJD, and RA have planned and designed the study. NM and SBM have collected the samples, conducted the experiment, and acquired the data. SK helped in sample collection. SV and TSG helped in data analysis. NM and RA wrote the manuscript and were responsible for proofreading and critical revision of the content. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mounika, N., Mungase, S.B., Verma, S. et al. Inflammatory Protein Signatures as Predictive Disease-Specific Markers for Non-Alcoholic Steatohepatitis (NASH). Inflammation (2024). https://doi.org/10.1007/s10753-024-02035-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02035-0