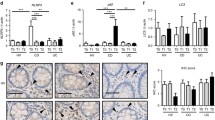
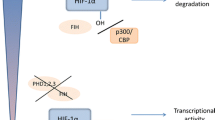
Abstract
In this study, we investigated the role of hypoxia in the development of chronic inflammatory bowel disease (IBD), focusing on its impact on the HIF-1α signaling pathway through the upregulation of lipocalin 2 (LCN2). Using a murine model of colitis induced by sodium dextran sulfate (DSS) under hypoxic conditions, transcriptome sequencing revealed LCN2 as a key gene involved in hypoxia-mediated exacerbation of colitis. Bioinformatics analysis highlighted the involvement of crucial pathways, including HIF-1α and glycolysis, in the inflammatory process. Immune infiltration analysis demonstrated the polarization of M1 macrophages in response to hypoxic stimulation. In vitro studies using RAW264.7 cells further elucidated the exacerbation of inflammation and its impact on M1 macrophage polarization under hypoxic conditions. LCN2 knockout cells reversed hypoxia-induced inflammatory responses, and the HIF-1α pathway activator dimethyloxaloylglycine (DMOG) confirmed LCN2's role in mediating inflammation via the HIF-1α-induced glycolysis pathway. In a DSS-induced colitis mouse model, oral administration of LCN2-silencing lentivirus and DMOG under hypoxic conditions validated the exacerbation of colitis. Evaluation of colonic tissues revealed altered macrophage polarization, increased levels of inflammatory factors, and activation of the HIF-1α and glycolysis pathways. In conclusion, our findings suggest that hypoxia exacerbates colitis by modulating the HIF-1α pathway through LCN2, influencing M1 macrophage polarization in glycolysis. This study contributes to a better understanding of the mechanisms underlying IBD, providing potential therapeutic targets for intervention.












Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Availability of Data and Materials
The data that supports the findings of this study are available on request from the corresponding author.
References
Upadhyay, K.G., D.C. Desai, T.F. Ashavaid, and A.J. Dherai. 2023. Microbiome and metabolome in inflammatory bowel disease. Journal of Gastroenterology and Hepatology 38 (1): 34–43. https://doi.org/10.1111/jgh.16043.
Zhang, Y.Z., and Y.Y. Li. 2014. Inflammatory bowel disease: Pathogenesis. World Journal of Gastroenterology 20 (1): 91–99. https://doi.org/10.3748/wjg.v20.i1.91.
Seyedian, S.S., F. Nokhostin, and M.D. Malamir. 2019. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. Journal of Medicine and Life 12 (2): 113–122. https://doi.org/10.25122/jml-2018-0075.
Franco, J.V.A., L.I. Garegnani, G.V. Oltra, M.I. Metzendorf, L.F. Trivisonno, N. Sgarbossa, D. Ducks, K. Heldt, R. Mumm, B. Barnes, and C. Scheidt-Nave. 2022. Long-Term Health Symptoms and Sequelae Following SARS-CoV-2 Infection: An Evidence Map. International Journal of Environmental Research And Public Health 19 (16): 9915. https://doi.org/10.3390/ijerph19169915.
Guan, Q. 2019. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. Journal of Immunology Research 2019: 7247238. https://doi.org/10.1155/2019/7247238.
Song, K., Y. Zhang, Q. Ga, Z. Bai, and R.L. Ge. 2020. High-altitude chronic hypoxia ameliorates obesity-induced non-alcoholic fatty liver disease in mice by regulating mitochondrial and AMPK signaling. Life Sciences 252: 117633. https://doi.org/10.1016/j.lfs.2020.117633.
Russel, M.G., and R.W. Stockbrügger. 2001. Epidemiologische ontwikkelingen en inzichten met betrekking tot chronische inflammatoire darmziekten [Epidemiological developments and insights in chronic inflammatory bowel diseases]. Nederlands tijdschrift voor geneeskunde 145 (30): 1448–1452.
Burtscher, J., R.T. Mallet, M. Burtscher, and G.P. Millet. 2021. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Research Reviews 68: 101343. https://doi.org/10.1016/j.arr.2021.101343.
Lun, J., H. Zhang, J. Guo, M. Yu, and J. Fang. 2023. Hypoxia inducible factor prolyl hydroxylases in inflammatory bowel disease. Frontiers in Pharmacology 14: 1045997. https://doi.org/10.3389/fphar.2023.1045997.
Singhal, R., and Y.M. Shah. 2020. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. The Journal of Biological Chemistry 295 (30): 10493–10505. https://doi.org/10.1074/jbc.REV120.011188.
Caio, G., L. Lungaro, F. Caputo, E. Zoli, F. Giancola, G. Chiarioni, R. De Giorgio, and G. Zoli. 2021. Nutritional Treatment in Crohn’s Disease. Nutrients 13 (5): 1628. https://doi.org/10.3390/nu13051628.
Scrimshaw, N.S., and E.B. Murray. 1988. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. The American Journal of Clinical Nutrition 48 (4 Suppl): 1079–1159. https://doi.org/10.1093/ajcn/48.4.1142.
Ballester Ferré, M.P., M.M. Boscá-Watts, and M. Mínguez Pérez. 2018. Crohn’s disease. Enfermedad de Crohn. Medicina Clinica 151 (1): 26–33. https://doi.org/10.1016/j.medcli.2017.10.036.
Glassner, K.L., B.P. Abraham, and E.M.M. Quigley. 2020. The microbiome and inflammatory bowel disease. The Journal of Allergy and Clinical Immunology 145 (1): 16–27. https://doi.org/10.1016/j.jaci.2019.11.003.
Lai, Y., X. Liang, H. Fan, Y. Liu, L. Zheng, W. Lu, Y. Sun, D. Huang, X. Liu, L. Zhang, D. Zuo, Z. Shou, Q. Tang, Y. Wang, Z. Li, Z. Jiang, S. Zang, H. Huang, Z. Tang, Q. Li, et al. 2022. Assessing the post-treatment therapeutic effect of tongxie in irritable bowel syndrome: A randomized controlled trial. Complementary Therapies in Medicine 68: 102839. https://doi.org/10.1016/j.ctim.2022.102839.
Sairenji, T., K.L. Collins, and D.V. Evans. 2017. An Update on Inflammatory Bowel Disease. Primary Care 44 (4): 673–692. https://doi.org/10.1016/j.pop.2017.07.010.
Zorgetto-Pinheiro, V.A., D.J. Machate, P.S. Figueiredo, G. Marcelino, P.A. Hiane, A. Pott, R.C.A. Guimarães, and D. Bogo. 2022. Omega-3 Fatty Acids and Balanced Gut Microbiota on Chronic Inflammatory Diseases: A Close Look at Ulcerative Colitis and Rheumatoid Arthritis Pathogenesis. Journal of Medicinal Food 25 (4): 341–354. https://doi.org/10.1089/jmf.2021.0012.
Liu, K., J. Yang, and H. Yuan. 2021. Recent progress in research on the gut microbiota and highland adaptation on the Qinghai-Tibet Plateau. Journal of Evolutionary Biology 34 (10): 1514–1530. https://doi.org/10.1111/jeb.13924.
Textor, S.C., and L.O. Lerman. 2019. The Role of Hypoxia in Ischemic Chronic Kidney Disease. Seminars in Nephrology 39 (6): 589–598. https://doi.org/10.1016/j.semnephrol.2019.10.008.
Rhodes, C.E., D. Denault, and M. Varacallo. 2022. Physiology. Oxygen Transport. In StatPearls: StatPearls Publishing.
Andersen, J.V., N.H. Skotte, S.K. Christensen, F.S. Polli, M. Shabani, K.H. Markussen, H. Haukedal, E.W. Westi, M. Diaz-delCastillo, R.C. Sun, K.A. Kohlmeier, A. Schousboe, M.S. Gentry, H. Tanila, K.K. Freude, B.I. Aldana, M. Mann, and H.S. Waagepetersen. 2021. Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death & Disease 12 (11): 954. https://doi.org/10.1038/s41419-021-04237-y.
Liu, H.M., H.Y. Tan, Y. Lin, B.N. Xu, W.H. Zhao, and Y.A. Xie. 2020. MicroRNA-1271-5p inhibits cell proliferation and enhances radiosensitivity by targeting CDK1 in hepatocellular carcinoma. Journal of Biochemistry 167 (5): 513–524. https://doi.org/10.1093/jb/mvz114.
Yan, R., L. Zhang, N. Xia, Q. Liu, H. Sun, and H. Guo. 2015. Knockdown of augmenter of liver regeneration in HK-2 cells inhibits inflammation response via the mitogen-activated protein kinase signaling pathway. Inflammation Research : Official Journal of the European Histamine Research Society 64 (6): 453–462. https://doi.org/10.1007/s00011-015-0825-x.
Zhang, Y., C. Guo, Y. Li, X. Han, X. Luo, L. Chen, T. Zhang, N. Wang, and W. Wang. 2022. Alginate Oligosaccharides Ameliorate DSS-Induced Colitis through Modulation of AMPK/NF-κB Pathway and Intestinal Microbiota. Nutrients 14 (14): 2864. https://doi.org/10.3390/nu14142864.
Shi, J., T. Yu, K. Song, S. Du, S. He, X. Hu, X. Li, H. Li, S. Dong, Y. Zhang, Z. Xie, C. Li, and J. Yu. 2021. Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway. Redox Biology 41: 101954. https://doi.org/10.1016/j.redox.2021.101954.
Ergin, S., N. Kherad, and M. Alagoz. 2022. RNA sequencing and its applications in cancer and rare diseases. Molecular Biology Reports 49 (3): 2325–2333. https://doi.org/10.1007/s11033-021-06963-0.
Deng, Y.J., E.H. Ren, W.H. Yuan, G.Z. Zhang, Z.L. Wu, and Q.Q. Xie. 2020. GRB10 and E2F3 as Diagnostic Markers of Osteoarthritis and Their Correlation with Immune Infiltration. Diagnostics (Basel, Switzerland) 10 (3): 171. https://doi.org/10.3390/diagnostics10030171.
Peng, X.Y., Y. Wang, H. Hu, X.J. Zhang, and Q. Li. 2019. Identification of the molecular subgroups in coronary artery disease by gene expression profiles. Journal of Cellular Physiology 234 (9): 16540–16548. https://doi.org/10.1002/jcp.28324.
Zhu, M., M. Ye, J. Wang, L. Ye, and M. Jin. 2020. Construction of Potential miRNA-mRNA Regulatory Network in COPD Plasma by Bioinformatics Analysis. International Journal of Chronic Obstructive Pulmonary Disease 15: 2135–2145. https://doi.org/10.2147/COPD.S255262.
Li, J., P. Zhao, Y. Tian, K. Li, L. Zhang, Q. Guan, X. Mei, and Y. Qin. 2021. The Anti-Inflammatory Effect of a Combination of Five Compounds From Five Chinese Herbal Medicines Used in the Treatment of COPD. Frontiers in Pharmacology 12: 709702. https://doi.org/10.3389/fphar.2021.709702.
Zeng, D., Z. Ye, R. Shen, G. Yu, J. Wu, Y. Xiong, R. Zhou, W. Qiu, N. Huang, L. Sun, X. Li, J. Bin, Y. Liao, M. Shi, and W. Liao. 2021. IOBR: Multi-Omics Immuno-Oncology Biological Research to Decode Tumor Microenvironment and Signatures. Frontiers in Immunology 12: 687975. https://doi.org/10.3389/fimmu.2021.687975.
Newman, A.M., C.L. Liu, M.R. Green, A.J. Gentles, W. Feng, Y. Xu, C.D. Hoang, M. Diehn, and A.A. Alizadeh. 2015. Robust enumeration of cell subsets from tissue expression profiles. Nature Methods 12 (5): 453–457. https://doi.org/10.1038/nmeth.3337.
Zeng, X., B. Yao, J. Liu, G.W. Gong, M. Liu, J. Li, H.F. Pan, Q. Li, D. Yang, P. Lu, D. Wu, P. Xu, B. Chen, P. Chen, M. Zhang, K. Zen, J. Jing, D.C.S. Huang, D. Chen, Z.W. Jiang, et al. 2023. The SMARCA4R1157W mutation facilitates chromatin remodeling and confers PRMT1/SMARCA4 inhibitors sensitivity in colorectal cancer. NPJ Precision Oncology 7 (1): 28. https://doi.org/10.1038/s41698-023-00367-y.
Rahimi, S., A.M. Roushandeh, A. Ebrahimi, A.A. Samadani, Y. Kuwahara, and M.H. Roudkenar. 2019. CRISPR/Cas9-mediated knockout of Lcn2 effectively enhanced CDDP-induced apoptosis and reduced cell migration capacity of PC3 cells. Life Sciences 231: 116586. https://doi.org/10.1016/j.lfs.2019.116586.
Gómez-Maldonado, L., M. Tiana, O. Roche, A. Prado-Cabrero, L. Jensen, A. Fernandez-Barral, I. Guijarro-Muñoz, E. Favaro, G. Moreno-Bueno, L. Sanz, J. Aragones, A. Harris, O. Volpert, B. Jimenez, and L. del Peso. 2015. EFNA3 long noncoding RNAs induced by hypoxia promote metastatic dissemination. Oncogene 34 (20): 2609–2620. https://doi.org/10.1038/onc.2014.200.
Liu, F., H. Qiu, M. Xue, S. Zhang, X. Zhang, J. Xu, J. Chen, Y. Yang, and J. Xie. 2019. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Research & Therapy 10 (1): 345. https://doi.org/10.1186/s13287-019-1447-y.
Sun, S., Y. Du, C. Yin, X. Suo, R. Wang, R. Xia, and X. Zhang. 2019. Water-separated part of Chloranthus serratus alleviates lipopolysaccharide- induced RAW264.7 cell injury mainly by regulating the MAPK and Nrf2/HO-1 inflammatory pathways. BMC Complementary and Alternative Medicine 19 (1): 343. https://doi.org/10.1186/s12906-019-2755-6.
Ying, M., D. You, X. Zhu, L. Cai, S. Zeng, and X. Hu. 2021. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biology 46: 102065. https://doi.org/10.1016/j.redox.2021.102065.
Jin, J., S. Qiu, P. Wang, X. Liang, F. Huang, H. Wu, B. Zhang, W. Zhang, X. Tian, R. Xu, H. Shi, and X. Wu. 2019. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. Journal of Experimental & Clinical Cancer Research : CR 38 (1): 377. https://doi.org/10.1186/s13046-019-1351-4.
Guo, Y., X. Jia, Y. Cui, Y. Song, S. Wang, Y. Geng, R. Li, W. Gao, and D. Fu. 2021. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biology 41: 101915. https://doi.org/10.1016/j.redox.2021.101915.
Gamah, M., M. Alahdal, Y. Zhang, Y. Zhou, Q. Ji, Z. Yuan, Y. Han, X. Shen, Y. Ren, and W. Zhang. 2021. High-altitude hypoxia exacerbates dextran sulfate sodium (DSS)-induced colitis by upregulating Th1 and Th17 lymphocytes. Bioengineered 12 (1): 7985–7994. https://doi.org/10.1080/21655979.2021.1975017.
Bian, J., F. Cai, H. Chen, Z. Tang, K. Xi, J. Tang, L. Wu, Y. Xu, L. Deng, Y. Gu, W. Cui, and L. Chen. 2021. Modulation of Local Overactive Inflammation via Injectable Hydrogel Microspheres. Nano Letters 21 (6): 2690–2698. https://doi.org/10.1021/acs.nanolett.0c04713.
Chen, M.L., C.G. Hong, T. Yue, H.M. Li, R. Duan, W.B. Hu, J. Cao, Z.X. Wang, C.Y. Chen, X.K. Hu, B. Wu, H.M. Liu, Y.J. Tan, J.H. Liu, Z.W. Luo, Y. Zhang, S.S. Rao, M.J. Luo, H. Yin, Y.Y. Wang, and Z.Z. Liu. 2021. Inhibition of miR-331–3p and miR-9–5p ameliorates Alzheimer’s disease by enhancing autophagy. Theranostics 11 (5): 2395–2409. https://doi.org/10.7150/thno.47408.
Wang, Y., Z. Wu, Y.T. Bai, G.Y. Wu, and G. Chen. 2017. Gad67 haploinsufficiency reduces amyloid pathology and rescues olfactory memory deficits in a mouse model of Alzheimer’s disease. Molecular Neurodegeneration 12 (1): 73. https://doi.org/10.1186/s13024-017-0213-9.
Zou, S., C. Wang, Z. Cui, P. Guo, Q. Meng, X. Shi, Y. Gao, G. Yang, and Z. Han. 2016. β-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacological Reports : PR 68 (1): 7–11. https://doi.org/10.1016/j.pharep.2015.06.004.
Liang, N., Y. Li, and H.Y. Chung. 2017. Two natural eudesmane-type sesquiterpenes from Laggera alata inhibit angiogenesis and suppress breast cancer cell migration through VEGF- and Angiopoietin 2-mediated signaling pathways. International Journal of Oncology 51 (1): 213–222. https://doi.org/10.3892/ijo.2017.4004.
Zhang, Q.F., J. Li, K. Jiang, R. Wang, J.L. Ge, H. Yang, S.J. Liu, L.T. Jia, L. Wang, and B.L. Chen. 2020. CDK4/6 inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics 10 (23): 10619–10633. https://doi.org/10.7150/thno.44871.
GBD 2017 Inflammatory Bowel Disease Collaborators. 2020. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Gastroenterology & Hepatology 5 (1): 17–30. https://doi.org/10.1016/S2468-1253(19)30333-4.
Fakhoury, M., R. Negrulj, A. Mooranian, and H. Al-Salami. 2014. Inflammatory bowel disease: Clinical aspects and treatments. Journal of Inflammation Research 7: 113–120. https://doi.org/10.2147/JIR.S65979.
Ott, A. 1987. Inflammation and transcutaneous measurement of oxygen pressure in dermatology. Advances in Experimental Medicine and Biology 220: 79–82. https://doi.org/10.1007/978-1-4613-1927-6_14.
Sawyer, R.G., M.D. Spengler, R.B. Adams, and T.L. Pruett. 1991. The peritoneal environment during infection. The effect of monomicrobial and polymicrobial bacteria on pO2 and pH. Annals of Surgery 213 (3): 253–260. https://doi.org/10.1097/00000658-199103000-00013.
Lippl, F.J., S. Neubauer, S. Schipfer, N. Lichter, A. Tufman, B. Otto, and R. Fischer. 2010. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring, Md.) 18 (4): 675–681. https://doi.org/10.1038/oby.2009.509.
Zheng, L., C.J. Kelly, and S.P. Colgan. 2015. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. American Journal of Physiology. Cell Physiology 309 (6): C350–C360. https://doi.org/10.1152/ajpcell.00191.2015.
Thibault, M.P., É. Tremblay, C. Horth, A. Fournier-Morin, D. Grynspan, C. Babakissa, E. Levy, E. Ferretti, V. Bertelle, and J.F. Beaulieu. 2022. Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates. Pediatric Research 91 (1): 129–136. https://doi.org/10.1038/s41390-021-01680-7.
Bakke, I., G.A. Walaas, T. Bruland, E.S. Røyset, A. van Beelen Granlund, C. Escudero-Hernández, S. Thorsvik, A. Münch, A.K. Sandvik, and A.E. Østvik. 2021. Mucosal and faecal neutrophil gelatinase-associated lipocalin as potential biomarkers for collagenous colitis. Journal of Gastroenterology 56 (10): 914–927. https://doi.org/10.1007/s00535-021-01814-y.
Na, Y.R., M. Stakenborg, S.H. Seok, and G. Matteoli. 2019. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nature reviews. Gastroenterology & Hepatology 16 (9): 531–543. https://doi.org/10.1038/s41575-019-0172-4.
Cheng, L., H. Xing, X. Mao, L. Li, X. Li, and Q. Li. 2015. Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model. Scandinavian Journal of Immunology 81 (1): 31–38. https://doi.org/10.1111/sji.12245.
Orecchioni, M., Y. Ghosheh, A.B. Pramod, and K. Ley. 2019. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs Classically and M2(LPS-) vs Alternatively Activated Macrophages. Frontiers in Immunology 10: 1084. https://doi.org/10.3389/fimmu.2019.01084.
Ciesielska, A., M. Matyjek, and K. Kwiatkowska. 2021. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cellular and Molecular Life Sciences : CMLS 78 (4): 1233–1261. https://doi.org/10.1007/s00018-020-03656-y.
Yunna, C., H. Mengru, W. Lei, and C. Weidong. 2020. Macrophage M1/M2 polarization. European Journal of Pharmacology 877: 173090. https://doi.org/10.1016/j.ejphar.2020.173090.
Griffiths, H.R., D. Gao, and C. Pararasa. 2017. Redox regulation in metabolic programming and inflammation. Redox Biology 12: 50–57. https://doi.org/10.1016/j.redox.2017.01.023.
Guma, M., S. Tiziani, and G.S. Firestein. 2016. Metabolomics in rheumatic diseases: Desperately seeking biomarkers. NatureReviews. Rheumatology 12 (5): 269–281. https://doi.org/10.1038/nrrheum.2016.1.
Zeng, W., Z. Xing, M. Tan, Y. Wu, and C. Zhang. 2021. Propofol regulates activated macrophages metabolism through inhibition of ROS-mediated GLUT1 expression. Inflammation research : Official Journal of the European Histamine Research Society 70 (4): 473–481. https://doi.org/10.1007/s00011-021-01449-y.
Zhong, H., R. Song, Q. Pang, Y. Liu, J. Zhuang, Y. Chen, J. Hu, J. Hu, Y. Liu, Z. Liu, and J. Tang. 2018. Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death & Disease 9 (10): 932. https://doi.org/10.1038/s41419-018-0996-9.
Ye, L., Y. Jiang, and M. Zhang. 2022. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine & Growth Factor Reviews 68: 81–92. https://doi.org/10.1016/j.cytogfr.2022.11.001.
Wang, G.L., B.H. Jiang, E.A. Rue, and G.L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America 92 (12): 5510–5514. https://doi.org/10.1073/pnas.92.12.5510.
Zhao, M., S. Wang, A. Zuo, J. Zhang, W. Wen, W. Jiang, H. Chen, D. Liang, J. Sun, and M. Wang. 2021. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cellular & Molecular Biology Letters 26 (1): 40. https://doi.org/10.1186/s11658-021-00283-8.
Semenza, G.L. 2012. Hypoxia-inducible factors in physiology and medicine. Cell 148 (3): 399–408. https://doi.org/10.1016/j.cell.2012.01.021.
Chen, Y., D. Zheng, H. Wang, S. Zhang, Y. Zhou, X. Ke, and G. Chen. 2023. Lipocalin 2 in the Paraventricular Thalamic Nucleus Contributes to DSS-Induced Depressive-Like Behaviors. Neuroscience Bulletin 39 (8): 1263–1277. https://doi.org/10.1007/s12264-023-01047-4.
Moschen, A.R., T.E. Adolph, R.R. Gerner, V. Wieser, and H. Tilg. 2017. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends in Endocrinology and Metabolism: TEM 28 (5): 388–397. https://doi.org/10.1016/j.tem.2017.01.003.
Xiao, X., B.S. Yeoh, and M. Vijay-Kumar. 2017. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annual Review of Nutrition 37: 103–130. https://doi.org/10.1146/annurev-nutr-071816-064559.
Du, D., C. Liu, M. Qin, X. Zhang, T. Xi, S. Yuan, H. Hao, and J. Xiong. 2022. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharmaceutica Sinica. B 12 (2): 558–580. https://doi.org/10.1016/j.apsb.2021.09.019.
Wu, Y.J., W.J. Fang, S. Pan, S.S. Zhang, D.F. Li, Z.F. Wang, W.G. Chen, Q. Yin, and J. Zuo. 2021. Regulation of Sirt1 on energy metabolism and immune response in rheumatoid arthritis. International Immunopharmacology 101 (Pt A): 108175. https://doi.org/10.1016/j.intimp.2021.108175.
Lu, J., M. Wang, Y. Chen, H. Song, D. Wen, J. Tu, Y. Guo, and Z. Liu. 2023. NAMPT inhibition reduces macrophage inflammation through the NAD+/PARP1 pathway to attenuate liver ischemia-reperfusion injury. Chemico-Biological interactions 369: 110294. https://doi.org/10.1016/j.cbi.2022.110294.
Ugarte, F., D. Santapau, V. Gallardo, C. Garfias, A. Yizmeyián, S. Villanueva, C. Sepúlveda, J. Rocco, C. Pasten, C. Urquidi, G. Cavada, P. San Martin, F. Cano, and C.E. Irarrázabal. 2022. Urinary Extracellular Vesicles as a Source of NGAL for Diabetic Kidney Disease Evaluation in Children and Adolescents With Type 1 Diabetes Mellitus. Frontiers in Endocrinology 12: 654269. https://doi.org/10.3389/fendo.2021.654269.
Bisgaard, T.H., K.H. Allin, L. Keefer, A.N. Ananthakrishnan, and T. Jess. 2022. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment Nature reviews. Gastroenterology & Hepatology 19 (11): 717–726. https://doi.org/10.1038/s41575-022-00634-6.
Kou, F., Y. Cheng, L. Shi, J. Liu, Y. Liu, R. Shi, G. Peng, and J. Li. 2022. LCN2 as a Potential Diagnostic Biomarker for Ulcerative Colitis-Associated Carcinogenesis Related to Disease Duration. Frontiers in Oncology 11: 793760. https://doi.org/10.3389/fonc.2021.793760.
Zhang, W., R. Pan, M. Lu, Q. Zhang, Z. Lin, Y. Qin, Z. Wang, S. Gong, H. Lin, S. Chong, L. Lu, W. Liao, and X. Lu. 2021. Epigenetic induction of lipocalin 2 expression drives acquired resistance to 5-fluorouracil in colorectal cancer through integrin β3/SRC pathway. Oncogene 40 (45): 6369–6380. https://doi.org/10.1038/s41388-021-02029-4.
Funding
This work was financially supported by the Cultivation Fund of the National Natural Science Foundation (Grant No. qiankehe2018-5764-11).
Author information
Authors and Affiliations
Contributions
YHY, FY and PSS wrote the paper and conceived and designed the experiments; LCY analyzed the data; DJC collected and provided the sample for this study. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All mouse experiments conducted in this study adhered to ethical principles and regulations governing the use of experimental animals, both internationally and domestically. The experimental procedures in this study have been approved by Guizhou Provincial People's Hospital, Medical College of Guizhou University (approval number: 2020413).
Consent to Participate
All participants in the study gave informed written consent to participate.
Consent for Publication
Authors: All authors have reviewed the manuscript and gave consent to publish.
Competing Interests
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Silencing efficiency verification of LCN2 sequence. Note: (A) RT-qPCR measured expression levels of LCN2 in mRNA of cells from different groups. (B) Expression levels of LCN2 in the protein of cells from different groups were detected by Western blot. The cell experiments were repeated three times. Compared to the sh-NC group, P<0.05, *P<0.01.(JPG 550 KB)
Fig. S2
Animal modeling process diagram. Note: Ctrl group: no intervention, normal drinking water; DSS group: after adaptive feeding, without any treatment, directly given 2.5% DSS concentration free drinking water for 7 days; DSS+hypoxia group: after adaptive feeding, without any treatment, directly given 2.5% DSS concentration free drinking water for 7 days, during which cultured in a hypoxic incubator. Transcriptional sequencing was performed on colonic tissues of the above three groups, and differential analysis was conducted between the Ctrl group vs DSS group and DSS group vs DSS+hypoxia group. DSS+hypoxia+Sh-NC group: targeted injection of empty lentivirus, after 7 days of recovery, given 2.5% DSS concentration free drinking water for 7 days, during which cultured in a hypoxic incubator; DSS+hypoxia+Sh-LCN2 group: targeted injection of LCN2-silencing lentivirus, after 7 days of recovery, given 2.5% DSS concentration free drinking water for 7 days, during which cultured in a hypoxic incubator; DSS+hypoxia+Sh-LCN2+DMOG group: targeted injection of LCN2-silencing lentivirus, after 7 days of recovery, given 2.5% DSS concentration free drinking water for 7 days, and daily administered 10mg/kg DMOG by gavage, during which cultured in a hypoxic incubator. Signs and symptoms were observed daily during the culture period, and biochemical tests were performed after euthanasia (JPG 3122 KB)
Fig. S3
The intersection of transcriptome sequencing results of two groups. Note: (A) Venn diagram of transcriptome sequencing results of two groups; (B) 12 differentially expressed genes in the intersection; (C) Heatmap showing the expression of the 12 differentially expressed genes in the two groups; (D) Interactions among the 12 differentially expressed genes. The LCN2 gene, a key gene identified in the previous analysis, is highlighted in red. (JPG 640 KB)
Fig. S4
CRISPR/Cas9 gene editing and knockout process of LCN2 and validation of knockout efficacy. Note: (A) Workflow of CRISPR/Cas9 gene editing and knockout of LCN2; (B) PCR products of LCN2-KO cells and β-actin from different groups run on agarose gel (Lane 1 = 100 bp ladder; Lane 2 = β-actin of RAW264.7-Scr cells; Lane 3 = β-actin of LCN2-KO cells; Lane 4 = LCN2 of RAW264.7-Scr cells; Lane 5 = LCN2 of LCN2-KO cells); (C) Immunofluorescence staining for the expression of LCN2. Compared to the KO-NC-scr group, P<0.05, *P<0.01. Bar=100 μm.(JPG 2726 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Yh., Yan, F., Shi, Ps. et al. HIF-1α Pathway Orchestration by LCN2: A Key Player in Hypoxia-Mediated Colitis Exacerbation. Inflammation 47, 1491–1519 (2024). https://doi.org/10.1007/s10753-024-01990-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-024-01990-y