Abstract
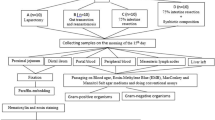
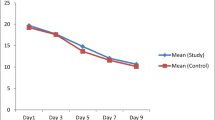
Many patients with ulcerative colitis suffer from malnutrition and intestinal flora disorders, which affect the postoperative intestinal barrier function of the ileal pouch. This study aimed to investigate the effects of enteral nutrition combined with probiotics after ileal pouch–anal anastomosis in rats. Male Sprague–Dawley rats underent ileal pouch–anal anastomosis and were randomly assigned to a control group (standard rat chow), enteral nutrition group (short-peptide enteral nutrition), or probiotic nutrition group (short-peptide enteral nutrition and Lactobacillus acidophilus). The primary outcomes were a histological score and occludin levels in the ileal pouch. The secondary outcomes were nutritional status and fecal flora distribution. The histological scores in the control group were significantly higher than in the enteral nutrition and probiotic nutrition groups (P < 0.05), while occludin levels were significantly lower in the controls compared with the other two groups (P < 0.05). Serum total protein, albumin, transthyretin, and transferrin levels were significantly higher in the probiotic nutrition group, followed by the enteral nutrition and control groups (all P < 0.05). Total fecal flora, and Gram-positive and Gram-negative rods differed significantly among the groups (all P < 0.05), but there were no significant differences in Gram-positive or Gram-negative cocci (all P > 0.05). Enteral nutrition combined with probiotics can effectively protect the intestinal barrier function of the ileal pouch in rats, possibly via the stable distribution of the intestinal flora and good nutritional status.







Similar content being viewed by others
Data Availability
Availability of data and material is not applicable for this study.
References
Iskandar, H.N., T. Dhere, and F.A. Farraye. 2015. Ulcerative colitis: update on medical management. Current Gastroenterology Reports 17: 44.
Bohl, J.L., and K. Sobba. 2015. Indications and options for surgery in ulcerative colitis. The Surgical Clinics of North America 95: 1211–1232 vi.
Durchschein, F., W. Petritsch, and H.F. Hammer. 2016. Diet therapy for inflammatory bowel diseases: the established and the new. World Journal of Gastroenterology 22: 2179–2194.
Luo, Z., J. Wang, Z. Zhang, et al. 2018. Efficacy of early enteral immunonutrition on immune function and clinical outcome for postoperative patients with gastrointestinal cancer. JPEN Journal of Parenteral and Enteral Nutrition 42: 758–765.
Peter, J.V., J.L. Moran, and J. Phillips-Hughes. 2005. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Critical Care Medicine 33: 213–220 discussion 260-261.
Sugita, T., S. Watarida, K. Katsuyama, et al. 1998. Endotoxemia after elective surgery for abdominal aortic aneurysm and the effect of early oral feeding. The Journal of Cardiovascular Surgery 39: 547–549.
Koopman, R., N. Crombach, A.P. Gijsen, S. Walrand, J. Fauquant, A.K. Kies, S. Lemosquet, W.H.M. Saris, Y. Boirie, and L.J.C. van Loon. 2009. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. The American Journal of Clinical Nutrition 90: 106–115.
Guslandi, M. 2015. Role of probiotics in Crohn’s disease and in pouchitis. Journal of Clinical Gastroenterology 49 (Suppl 1): S46–S49.
Lichtenstein, L., I. Avni-Biron, and O. Ben-Bassat. 2016. The current place of probiotics and prebiotics in the treatment of pouchitis. Best Practice & Research. Clinical Gastroenterology 30: 73–80.
Gosselink, M.P., W.R. Schouten, L.M. van Lieshout, W.C. Hop, J.D. Laman, and J.G. Ruseler-van Embden. 2004. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Diseases of the Colon and Rectum 47: 876–884.
Sood, A., V. Midha, G.K. Makharia, et al. 2009. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clinical Gastroenterology and Hepatology 7: 1202–1209 1209.e1.
Gionchetti, P., C. Calabrese, A. Lauri, and F. Rizzello. 2015. The therapeutic potential of antibiotics and probiotics in the treatment of pouchitis. Expert Review of Gastroenterology & Hepatology 9: 1175–1181.
Pronio, A., C. Montesani, C. Butteroni, S. Vecchione, G. Mumolo, A.R. Vestri, D. Vitolo, and M. Boirivant. 2008. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflammatory Bowel Diseases 14: 662–668.
El Sayed, N.S., and A.S. Sayed. 2019. Protective effect of methylene blue on TNBS-induced colitis in rats mediated through the modulation of inflammatory and apoptotic signalling pathways. Archives of Toxicology 93: 2927–2942.
Lewis, S.J., and K.W. Heaton. 1997. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology 32: 920–924.
Shebani, K.O., A.F. Stucchi, B. Fruin, J.P. McClung, D. Gee, E.R. Beer, W.W. LaMorte, and J.M. Becker. 2002. Pouchitis in a rat model of ileal J pouch-anal anastomosis. Inflammatory Bowel Diseases 8: 23–34.
Atila, K., C. Terzi, A.E. Canda, S.T. Akhisaroglu, H.S. Avci, S. Sarioglu, G. Oktay, and Z. Gulay. 2009. Partially hydrolyzed guar gum attenuates the severity of pouchitis in a rat model of ileal J pouch-anal anastomosis. Digestive Diseases and Sciences 54: 522–529.
Csontos, Á.A., A. Molnár, Z. Piri, E. Pálfi, and P. Miheller. 2017. Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease. Revista Española de Enfermedades Digestivas 109: 26–32.
Ma, X., Y. Chen, F. Huang, Q. Luo, H. Lv, and H. Long. 2016. Food intolerance prevalence in active ulcerative colitis in Southwest China. Asia Pacific Journal of Clinical Nutrition 25: 529–533.
Jeppesen, P.B., B. Hartmann, J. Thulesen, J. Graff, J. Lohmann, B.S. Hansen, F. Tofteng, S.S. Poulsen, J.L. Madsen, J.J. Holst, and P.B. Mortensen. 2001. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815.
Preidis, G.A., D.M. Saulnier, S.E. Blutt, T.A. Mistretta, K.P. Riehle, A.M. Major, S.F. Venable, J.P. Barrish, M.J. Finegold, J.F. Petrosino, R.L. Guerrant, M.E. Conner, and J. Versalovic. 2012. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. Journal of Pediatric Gastroenterology and Nutrition 55: 299–307.
Currò, D., G. Ianiro, S. Pecere, S. Bibbò, and G. Cammarota. 2017. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. British Journal of Pharmacology 174: 1426–1449.
Caporaso, J.G., C.L. Lauber, E.K. Costello, D. Berg-Lyons, A. Gonzalez, J. Stombaugh, D. Knights, P. Gajer, J. Ravel, N. Fierer, J.I. Gordon, and R. Knight. 2011. Moving pictures of the human microbiome. Genome Biology 12: R50.
Ganji-Arjenaki, M., and M. Rafieian-Kopaei. 2018. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. Journal of Cellular Physiology 233: 2091–2103.
Collado, M.C., J. Meriluoto, and S. Salminen. 2007. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Letters in Applied Microbiology 45: 454–460.
Kotzampassi, K., and E.J. Giamarellos-Bourboulis. 2012. Probiotics for infectious diseases: more drugs, less dietary supplementation. International Journal of Antimicrobial Agents 40: 288–296.
Kanauchi, O., Y. Matsumoto, M. Matsumura, M. Fukuoka, and T. Bamba. 2005. The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Current Pharmaceutical Design 11: 1047–1053.
Singh, S., A.M. Stroud, S.D. Holubar, W.J. Sandborn, and D.S. Pardi. 2015. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database of Systematic Reviews 5: CD001176.
Macho Fernandez, E., B. Pot, and C. Grangette. 2011. Beneficial effect of probiotics in IBD: are peptidogycan and NOD2 the molecular key effectors. Gut Microbes 2: 280–286.
Devoto, G., F. Gallo, C. Marchello, O. Racchi, R. Garbarini, S. Bonassi, G. Albalustri, and E. Haupt. 2006. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clinical Chemistry 52: 2281–2285.
Wang, F., M.X. Hou, X.L. Wu, L.D. Bao, and P.D. Dong. 2015. Impact of enteral nutrition on postoperative immune function and nutritional status. Genetics and Molecular Research 14: 6065–6072.
Brugler, L., A. Stankovic, L. Bernstein, F. Scott, and J. O’Sullivan-Maillet. 2002. The role of visceral protein markers in protein calorie malnutrition. Clinical Chemistry and Laboratory Medicine 40: 1360–1369.
López-Hellin, J., J.A. Baena-Fustegueras, S. Schwartz-Riera, and E. García-Arumí. 2002. Usefulness of short-lived proteins as nutritional indicators surgical patients. Clinical Nutrition 21: 119–125.
Seres, D.S., and P.R. Ippolito. 2017. Pilot study evaluating the efficacy, tolerance and safety of a peptide-based enteral formula versus a high protein enteral formula in multiple ICU settings (medical, surgical, cardiothoracic). Clinical Nutrition 36: 706–709.
Gionchetti, P., F. Rizzello, and M. Campieri. 2002. Probiotics in gastroenterology. Current Opinion in Gastroenterology 18: 235–239.
Yasueda, A., T. Mizushima, R. Nezu, R. Sumi, M. Tanaka, J. Nishimura, Y. Kai, M. Hirota, H. Osawa, K. Nakajima, M. Mori, and T. Ito. 2016. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surgery Today 46: 939–949.
Hudson, L.E., S.E. Anderson, A.H. Corbett, and T.J. Lamb. 2017. Gleaning insights from fecal microbiota transplantation and probiotic studies for the rational design of combination microbial therapies. Clinical Microbiology Reviews 30: 191–231.
Beura, L.K., S.E. Hamilton, K. Bi, J.M. Schenkel, O.A. Odumade, K.A. Casey, E.A. Thompson, K.A. Fraser, P.C. Rosato, A. Filali-Mouhim, R.P. Sekaly, M.K. Jenkins, V. Vezys, W.N. Haining, S.C. Jameson, and D. Masopust. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516.
Acknowledgments
We thank Susan Furness, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Code availability
Code availability is not applicable for this study.
Funding
This work was supported by a grant from the Li Jie-shou Gut Barrier Foundation (Grant No. LJS_201008).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yanyan Xu, Zongran Chen, and Xin Wang. The first draft of the manuscript was written by Ting Zheng and Ying Gao. Jian Liu and Gang Liu revised it critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The experiments were reviewed and approved by the Animal Ethics and Welfare Committee (approval number: TMUaMEC2017001).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Zheng and Ying Gao contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Zheng, T., Gao, Y., Xu, Y. et al. Combination of Enteral Nutrition and Probiotics Promote Recovery Following Ileal Pouch–Anal Anastomosis in Rats. Inflammation 44, 725–736 (2021). https://doi.org/10.1007/s10753-020-01372-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01372-0