Abstract
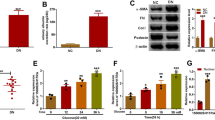
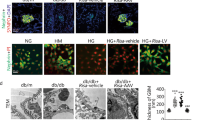
Diabetic nephropathy (DN), characterized by glomerular injury, is a common complication of both type 1 and type 2 diabetes, accompanied by massive proteinuria. Podocytes are reported to play pivotal roles in maintaining the glomerular filtration barrier. In addition, the expression of long non-coding RNAs (lncRNAs) ANRIL was upregulated in type 2 diabetes patients. Hence, the aim of this study was to investigate the underlying mechanisms implicated the role of LncRNA ANRIL in podocyte injury in DN. The concentration of inflammatory cytokines was quantified by the corresponding enzyme-linked immunosorbent assay (ELISA) kits. The mRNA levels of the target gene were determined by reverse transcription and real-time quantitative PCR (RT-qPCR). The expressions of proteins were evaluated by Western blot. The activities of lactate dehydrogenase (LDH), superoxide dismutase (SOD), and malondialdehyde (MDA) level were measured by corresponding commercial kits. Finally, the apoptosis of podocytes was analyzed by TUNEL assay. In our study, LncRNA ANRIL was highly expressed in high glucose (HG)-induced podocytes. Moreover, LncRNA ANRIL silencing attenuated HG-induced inflammation, oxidative stress, and apoptosis and induced MME overexpression in podocytes. Interestingly, MME knockdown abolished the suppressive effect of LncRNA ANRIL silencing on HG-induced inflammation, oxidative stress, and apoptosis in podocytes. LncRNA ANRIL silencing alleviates HG-induced inflammation, oxidative stress, and apoptosis via upregulation of MME in podocytes. Hence, LncRNA ANRIL may be a novel and effective target to ameliorate podocyte injury in DN.





Similar content being viewed by others
Abbreviations
- DN:
-
diabetic nephropathy
- ESRD:
-
end-stage renal disease
- LncRNAs:
-
long non-coding RNAs
- TNF:
-
tumor necrosis factor
- IL:
-
interleukin
- LDH:
-
lactate dehydrogenase
- SOD:
-
superoxide dismutase
- MDA:
-
malondialdehyde
- HG:
-
high glucose
- MME:
-
membrane metallo-endopeptidase
References
Gnudi, L., R.J.M. Coward, and D.A. Long. 2016. Diabetic nephropathy: Perspective on novel molecular mechanisms. Trends in Endocrinology and Metabolism 27: 820–830.
Yamahara, K., M. Yasuda, S. Kume, D. Koya, H. Maegawa, and T. Uzu. 2013. The role of autophagy in the pathogenesis of diabetic nephropathy. Journal Diabetes Research 2013: 193757.
Gheith, O., N. Farouk, N. Nampoory, M.A. Halim, and T. Al-Otaibi. 2016. Diabetic kidney disease: world wide difference of prevalence and risk factors. Journal Nephropharmacol 5: 49–56.
Feng, Y., H. Weng, L. Ling, T. Zeng, Y. Zhang, D. Chen, and H. Li. 2019. Modulating the gut microbiota and inflammation is involved in the effect of bupleurum polysaccharides against diabetic nephropathy in mice. International Journal of Biological Macromolecules 132: 1001–1011.
Zhang, P., J. Fang, J. Zhang, S. Ding, and D. Gan. 2020. Curcumin inhibited podocyte cell apoptosis and accelerated cell autophagy in diabetic nephropathy via regulating Beclin1/UVRAG/Bcl2. Diabetes Metabolic Syndrome and Obesity 13: 641–652.
Kim, Y.I., C.H. Kim, C.S. Choi, Y.E. Chung, M.S. Lee, S.I. Lee, J.Y. Park, S.K. Hong, and K.U. Lee. 2001. Microalbuminuria is associated with the insulin resistance syndrome independent of hypertension and type 2 diabetes in the Korean population. Diabetes Research and Clinical Practice 52: 145–152.
Al-Rubeaan, K., K. Siddiqui, M. Alghonaim, A.M. Youssef, and D. AlNaqeb. 2018. The Saudi Diabetic Kidney Disease Study (Saudi-DKD): clinical characteristics and biochemical parameters. Annals of Saudi Medicine 38: 46–56.
Yasuda-Yamahara, M., S. Kume, A. Tagawa, H. Maegawa, and T. Uzu. 2015. Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy 11: 2385–2386.
Lin, J.S., and K. Susztak. 2016. Podocytes: the weakest link in diabetic kidney disease? Current Diabetes Reports 16: 45.
Tagawa, A., M. Yasuda, S. Kume, K. Yamahara, J. Nakazawa, M. Chin-Kanasaki, H. Araki, S. Araki, D. Koya, K. Asanuma, E.H. Kim, M. Haneda, N. Kajiwara, K. Hayashi, H. Ohashi, S. Ugi, H. Maegawa, and T. Uzu. 2016. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65: 755–767.
Ziyadeh, F.N., and G. Wolf. 2008. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Current Diabetes Reviews 4: 39–45.
Liu, B.C., X. Song, X.Y. Lu, D.T. Li, D.C. Eaton, B.Z. Shen, X.Q. Li, and H.P. Ma. 2013. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochimica et Biophysica Acta 1833: 1434–1442.
Zhan, X., C. Yan, Y. Chen, X. Wei, J. Xiao, L. Deng, Y. Yang, P. Qiu, and Q. Chen. 2018. Celastrol antagonizes high glucose-evoked podocyte injury, inflammation and insulin resistance by restoring the HO-1-mediated autophagy pathway. Molecular Immunology 104: 61–68.
Li, S., X. Liu, J. Lei, J. Yang, P. Tian, and Y. Gao. 2017. Crocin protects podocytes against oxidative stress and inflammation induced by high glucose through inhibition of NF-kappaB. Cellular Physiology and Biochemistry 42: 1481–1492.
Chen, Y., Q. Liu, Z. Shan, Y. Zhao, M. Li, B. Wang, X. Zheng, and W. Feng. 2019. The protective effect and mechanism of catalpol on high glucose-induced podocyte injury. BMC Complementary and Alternative Medicine 19: 244.
Morris, K.V., and J.S. Mattick. 2014. The rise of regulatory RNA. Nature Reviews. Genetics 15: 423–437.
Sathishkumar, C., P. Prabu, V. Mohan, and M. Balasubramanyam. 2018. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Human Genomics 12: 41.
Thomas, A.A., B. Feng, and S. Chakrabarti. 2018. ANRIL regulates production of extracellular matrix proteins and vasoactive factors in diabetic complications. American Journal of Physiology. Endocrinology and Metabolism 314: E191–E200.
Lupfer, C., P.G. Thomas, P.K. Anand, P. Vogel, S. Milasta, J. Martinez, G. Huang, M. Green, M. Kundu, H. Chi, R.J. Xavier, D.R. Green, M. Lamkanfi, C.A. Dinarello, P.C. Doherty, and T.D. Kanneganti. 2013. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nature Immunology 14: 480–488.
Morigi, M., L. Perico, D. Corna, M. Locatelli, P. Cassis, C.E. Carminati, S. Bolognini, C. Zoja, G. Remuzzi, A. Benigni, and S. Buelli. 2020. C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight 5.
Gagliardini, E., N. Perico, P. Rizzo, S. Buelli, L. Longaretti, L. Perico, S. Tomasoni, C. Zoja, D. Macconi, M. Morigi, G. Remuzzi, and A. Benigni. 2013. Angiotensin II contributes to diabetic renal dysfunction in rodents and humans via notch1/snail pathway. The American Journal of Pathology 183: 119–130.
Wolf, G., S. Chen, and F.N. Ziyadeh. 2005. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634.
Li, M., Q. Guo, H. Cai, H. Wang, Z. Ma, and X. Zhang. 2020. miR-218 regulates diabetic nephropathy via targeting iKK-beta and modulating NK-kappaB-mediated inflammation. Journal of Cellular Physiology 235: 3362–3371.
Yu, Q., M. Zhang, L. Qian, D. Wen, and G. Wu. 2019. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sciences 225: 1–7.
Hou, Y., S. Lin, J. Qiu, W. Sun, M. Dong, Y. Xiang, L. Wang, and P. Du. 2020. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochemical and Biophysical Research Communications 521: 791–798.
Hu, J., H. Wu, D. Wang, Z. Yang, and J. Dong. 2019. LncRNA ANRIL promotes nlrp3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie 157: 102–110.
Wei, J.C., Y.L. Shi, and Q. Wang. 2019. LncRNA ANRIL knockdown ameliorates retinopathy in diabetic rats by inhibiting the NF-kappaB pathway. European Review for Medical and Pharmacological Sciences 23: 7732–7739.
Wang, Y.Z., W.W. Xu, D.Y. Zhu, N. Zhang, Y.L. Wang, M. Ding, X.M. Xie, L.L. Sun, and X.X. Wang. 2018. Specific expression network analysis of diabetic nephropathy kidney tissue revealed key methylated sites. Journal of Cellular Physiology 233: 7139–7147.
Ramirez, A.K., S. Dankel, W. Cai, M. Sakaguchi, S. Kasif, and C.R. Kahn. 2019. Membrane metallo-endopeptidase (neprilysin) regulates inflammatory response and insulin signaling in white preadipocytes. Molecular Metabolism 22: 21–36.
Liu, X.L., J.L. Liu, Y.C. Xu, X. Zhang, Y.X. Wang, L.H. Qing, W. Guo, J. Ding, and L.H. Meng. 2019. Membrane metallo-endopeptidase mediates cellular senescence induced by oncogenic PIK3CA(H1047R) accompanied with pro-tumorigenic secretome. International Journal of Cancer 145: 817–829.
Guo, Z., L. Li, Y. Gao, X. Zhang, and M. Cheng. 2019. Overexpression of lncRNA ANRIL aggravated hydrogen peroxide-disposed injury in pc-12 cells via inhibiting miR-499a/PDCD4 axis-mediated PI3K/Akt/mTOR/p70S6K pathway. Artificial Cells Nanomedicine Biotechnology 47: 2624–2633.
Author information
Authors and Affiliations
Contributions
R.C. and J.J. participated in the conception of the study and writing of the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, R., Jiang, J. LncRNA ANRIL Silencing Alleviates High Glucose-Induced Inflammation, Oxidative Stress, and Apoptosis via Upregulation of MME in Podocytes. Inflammation 43, 2147–2155 (2020). https://doi.org/10.1007/s10753-020-01282-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01282-1