Abstract
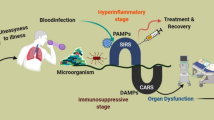
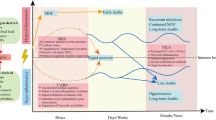
Sepsis is a major health problem all over the world. Despite its existence since the time of Hippocrates (470 BC), sepsis is still a serious medical problem for physicians working in both pediatric and adult intensive care units. The most current US FDA-approved drug called recombinant human activated protein C or Drotrecogin-α is also failed in clinical trials and showed similar effects as placebo. The epidemiological data and studies have indicated sepsis as a major socioeconomic burden all over the world. Advances in immunology and genomic medicine have established different immunological mechanisms as major regulators of the pathogenesis of the sepsis. These immunological mechanisms come into action upon activation of several components of the immune system including innate and adaptive immunity. The activation of these immune cells in response to the pathogens or pathogen-associated molecular patterns (PAMPs) responsible for the onset of sepsis is regulated by the metabolic stage of the immune cells called immunometabolism. An alternation in the immunometabolism is responsible for the generation of dysregulated immune response during sepsis and plays a very important role in the process. Thus, it becomes vital to understand the immunometabolic reprograming during sepsis to design future target-based therapeutics depending on the severity. The current review is designed to highlight the importance of immune response and associated immunometabolism during sepsis and its targeting as a future therapeutic approach.





Similar content being viewed by others
Change history
09 February 2019
The original version of this article contained mistakes, and the authors would like to correct them. The correct details are given below: In the published article, the subheading “Immunometabolic Reprogramming Among MDSCs During Sepsis” should read as “Immunometabolic Reprogramming Among Endothelial Cells or ECs During Sepsis”.
References
Singer, M., C.S. Deutschman, C. Seymour, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama 315: 801–810.
Angus, D.C., and T. van der Poll. 2013. Severe sepsis and septic shock. New England Journal of Medicine 369: 840–851.
Shankar-Hari, M., G.S. Phillips, M.L. Levy, et al. 2016. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). Jama 315: 775–787.
Marik, P.E., W.T. Linde-Zwirble, E.A. Bittner, J. Sahatjian, and D. Hansell. 2017. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Medicine 43: 625–632.
Rhodes, A., L.E. Evans, W. Alhazzani, M.M. Levy, M. Antonelli, R. Ferrer, A. Kumar, J.E. Sevransky, C.L. Sprung, M.E. Nunnally, B. Rochwerg, G.D. Rubenfeld, D.C. Angus, D. Annane, R.J. Beale, G.J. Bellinghan, G.R. Bernard, J.D. Chiche, C. Coopersmith, D.P. De Backer, C.J. French, S. Fujishima, H. Gerlach, J.L. Hidalgo, S.M. Hollenberg, A.E. Jones, D.R. Karnad, R.M. Kleinpell, Y. Koh, T.C. Lisboa, F.R. Machado, J.J. Marini, J.C. Marshall, J.E. Mazuski, L.A. McIntyre, A.S. McLean, S. Mehta, R.P. Moreno, J. Myburgh, P. Navalesi, O. Nishida, T.M. Osborn, A. Perner, C.M. Plunkett, M. Ranieri, C.A. Schorr, M.A. Seckel, C.W. Seymour, L. Shieh, K.A. Shukri, S.Q. Simpson, M. Singer, B.T. Thompson, S.R. Townsend, T. Van der Poll, J.L. Vincent, W.J. Wiersinga, J.L. Zimmerman, and R.P. Dellinger. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine 43: 304–377.
Taeb, A.M., M.H. Hooper, and P.E. Marik. 2017. Sepsis: current definition, pathophysiology, diagnosis, and management. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition 32: 296–308.
Vincent, J.L., Y. Sakr, C.L. Sprung, V.M. Ranieri, K. Reinhart, H. Gerlach, R. Moreno, J. Carlet, J.R. Le Gall, and D. Payen. 2006. Sepsis in European intensive care units: results of the SOAP study. Critical Care Medicine 34: 344–353.
Fleischmann, C., A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos, P. Schlattmann, D.C. Angus, and K. Reinhart. 2016a. Assessment of global incidence and mortality of hospital-treated sepsis. Current Estimates and Limitations. American journal of respiratory and critical care medicine 193: 259–272.
Fleischmann, C., D.O. Thomas-Rueddel, M. Hartmann, C.S. Hartog, T. Welte, S. Heublein, U. Dennler, and K. Reinhart. 2016b. Hospital incidence and mortality rates of sepsis. Deutsches Arzteblatt international 113: 159–166.
Cantey, J.B., Milstone, A.M., 2015. Bloodstream infections: epidemiology and resistance. Clinics in Perinatology 42, 1–16, vii.
Mohsen, L., N. Ramy, D. Saied, D. Akmal, N. Salama, M.M. Abdel Haleim, and H. Aly. 2017. Emerging antimicrobial resistance in early and late-onset neonatal sepsis. Antimicrobial Resistance and Infection Control 6: 63.
Pradipta, I.S., D.C. Sodik, K. Lestari, I. Parwati, E. Halimah, A. Diantini, and R. Abdulah. 2013. Antibiotic resistance in sepsis patients: evaluation and recommendation of antibiotic use. North American Journal of Medical Sciences 5: 344–352.
Esposito, S., G. De Simone, G. Boccia, F. De Caro, and P. Pagliano. 2017. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. Journal of Global Antimicrobial Resistance 10: 204–212.
Keener, A.B. 2017. Host with the most: targeting host cells instead of pathogens to fight infectious disease. Nature Medicine 23: 528–531.
Al-Khami, A.A., P.C. Rodriguez, and A.C. Ochoa. 2016. Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. Oncoimmunology 5: e1200771.
Beezhold, K., and C.A. Byersdorfer. 2018. Targeting immuno-metabolism to improve anti-cancer therapies. Cancer Letters 414: 127–135.
Bettencourt, I.A., and J.D. Powell. 2017. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. Journal of immunology (Baltimore, Md. : 1950) 198: 999–1005.
McKinney, E.F., and K.G.C. Smith. 2018. Metabolic exhaustion in infection, cancer and autoimmunity. Nature Immunology 19: 213–221.
Al-Khami, A.A., P.C. Rodriguez, and A.C. Ochoa. 2017. Energy metabolic pathways control the fate and function of myeloid immune cells. Journal of Leukocyte Biology 102: 369–380.
Dühring, S., S. Germerodt, C. Skerka, P. Zipfel, T. Dandekar, and S. Schuster. 2015. Host-pathogen interactions between the human innate immune system and Candida albicans—understanding and modeling defense and evasion strategies. Frontiers in Microbiology 6.
Kumar, H., T. Kawai, and S. Akira. 2011. Pathogen recognition by the innate immune system. International Reviews of Immunology 30: 16–34.
van der Poll, T., and S.M. Opal. 2008b. Host–pathogen interactions in sepsis. The Lancet Infectious Diseases 8: 32–43.
Kan, B., H.R. Razzaghian, and P.M. Lavoie. 2016. An immunological perspective on neonatal sepsis. Trends in Molecular Medicine 22: 290–302.
Kollmann, T.R., O. Levy, R.R. Montgomery, and S. Goriely. 2012. Innate immune function by toll-like receptors: distinct responses in newborns and the elderly. Immunity 37: 771–783.
Yost, C.C., M.J. Cody, E.S. Harris, N.L. Thornton, A.M. McInturff, M.L. Martinez, N.B. Chandler, C.K. Rodesch, K.H. Albertine, C.A. Petti, A.S. Weyrich, and G.A. Zimmerman. 2009. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113: 6419–6427.
Gomez, C.R., E.D. Boehmer, and E.J. Kovacs. 2005. The aging innate immune system. Current Opinion in Immunology 17: 457–462.
Montecino-Rodriguez, E., B. Berent-Maoz, and K. Dorshkind. 2013. Causes, consequences, and reversal of immune system aging. The Journal of Clinical Investigation 123: 958–965.
Shaw, A.C., S. Joshi, H. Greenwood, A. Panda, and J.M. Lord. 2010. Aging of the innate immune system. Current Opinion in Immunology 22: 507–513.
Solana, R., R. Tarazona, I. Gayoso, O. Lesur, G. Dupuis, and T. Fulop. 2012. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Seminars in Immunology 24: 331–341.
Kumar, V., and A. Sharma. 2008. Innate immunity in sepsis pathogenesis and its modulation: new immunomodulatory targets revealed. Journal of chemotherapy (Florence, Italy) 20: 672–683.
van der Poll, T., and S.M. Opal. 2008a. Host-pathogen interactions in sepsis. The Lancet. Infectious diseases 8: 32–43.
van der Poll, T., F.L. van de Veerdonk, B.P. Scicluna, and M.G. Netea. 2017. The immunopathology of sepsis and potential therapeutic targets. Nature Reviews. Immunology 17: 407–420.
Weber, G.F., and F.K. Swirski. 2014. Immunopathogenesis of abdominal sepsis. Langenbeck's Archives of Surgery 399: 1–9.
Wiersinga, W.J., S.J. Leopold, D.R. Cranendonk, and T. van der Poll. 2014. Host innate immune responses to sepsis. Virulence 5: 36–44.
Censoplano, N., C.L. Epting, and B.M. Coates. 2014. The role of the innate immune system in sepsis. Clinical Pediatric Emergency Medicine 15: 169–176.
Charchaflieh, J., J. Wei, G. Labaze, Y.J. Hou, B. Babarsh, H. Stutz, H. Lee, S. Worah, and M. Zhang. 2012. The role of complement system in septic shock. Clinical and Developmental Immunology 2012: 8.
Markiewski, M.M., R.A. DeAngelis, and J.D. Lambris. 2008. Complexity of complement activation in sepsis. Journal of Cellular and Molecular Medicine 12: 2245–2254.
Al-Soudi, A., M.H. Kaaij, and S.W. Tas. 2017. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmunity Reviews 16: 951–962.
Bell, E. 2009. Endothelial cells as sentinels. Nature Reviews Immunology 9: 532.
Mai, J., A. Virtue, J. Shen, H. Wang, and X.-F. Yang. 2013. An evolving new paradigm: endothelial cells—conditional innate immune cells. Journal of Hematology & Oncology 6: 61–61.
Andonegui, G., H. Zhou, D. Bullard, M.M. Kelly, S.C. Mullaly, B. McDonald, E.M. Long, S.M. Robbins, and P. Kubes. 2009. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. The Journal of Clinical Investigation 119: 1921–1930.
Aird, W.C. 2003. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101: 3765–3777.
Boisrame-Helms, J., H. Kremer, V. Schini-Kerth, and F. Meziani. 2013. Endothelial dysfunction in sepsis. Current Vascular Pharmacology 11: 150–160.
Ince, C., P.R. Mayeux, T. Nguyen, H. Gomez, J.A. Kellum, G.A. Ospina-Tascón, G. Hernandez, P. Murray, and D. De Backer. 2016. THE ENDOTHELIUM IN SEPSIS, Shock (Augusta, Ga.). 45: 259–270.
Opal, S.M., and T. van der Poll. 2015. Endothelial barrier dysfunction in septic shock. Journal of Internal Medicine 277: 277–293.
Peters, K., R.E. Unger, J. Brunner, and C.J. Kirkpatrick. 2003. Molecular basis of endothelial dysfunction in sepsis. Cardiovascular Research 60: 49–57.
Vallet, B. 2003. Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction? Critical Care 7: 130–138.
Chaudhry, H., Zhou, J., Zhong, Y.I.N., Ali, M.M., McGuire, F., Nagarkatti, P.S., Nagarkatti, M., 2013. Role of cytokines as a double-edged sword in sepsis. In vivo (Athens, Greece) 27, 669-684.
Chousterman, B.G., F.K. Swirski, and G.F. Weber. 2017. Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology 39: 517–528.
Tisoncik, J.R., M.J. Korth, C.P. Simmons, J. Farrar, T.R. Martin, and M.G. Katze. 2012. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews 76: 16–32.
Gogos, C.A., E. Drosou, H.P. Bassaris, and A. Skoutelis. 2000. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. The Journal of Infectious Diseases 181: 176–180.
Schulte, W., J. Bernhagen, and R. Bucala. 2013. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators of Inflammation 2013: 16.
Wang, D.-W., N. Dong, Y. Wu, X.-M. Zhu, C.-T. Wang, and Y.-M. Yao. 2016b. Interleukin-37 enhances the suppressive activity of naturally occurring CD4+CD25+ regulatory T cells. Scientific Reports 6: 38955.
Yan, J., A. Mitra, J. Hu, J.J. Cutrera, X. Xia, T. Doetschman, M. Gagea, L. Mishra, and S. Li. 2016. IL-30 (IL27p28) alleviates sepsis via modulation of cytokine profiles produced by NKT cells. Journal of Hepatology 64: 1128–1136.
Gerlach, H., 2016. Agents to reduce cytokine storm [version 1; referees: 3 approved].
Darenberg, J., N. Ihendyane, J. Sjolin, E. Aufwerber, S. Haidl, P. Follin, J. Andersson, and A. Norrby-Teglund. 2003. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 37: 333–340.
Shah, S.S., M. Hall, R. Srivastava, A. Subramony, and J.E. Levin. 2009. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49: 1369–1376.
Valiquette, L., D.E. Low, and A.J. McGeer. 2009. Assessing the impact of intravenous immunoglobulin in the management of streptococcal toxic shock syndrome: a noble but difficult quest. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49: 1377–1379.
Marshall, J.C. 2014. Why have clinical trials in sepsis failed? Trends in Molecular Medicine 20: 195–203.
Opal, S.M., P.F. Laterre, B. Francois, S.P. LaRosa, D.C. Angus, J.P. Mira, X. Wittebole, T. Dugernier, D. Perrotin, M. Tidswell, L. Jauregui, K. Krell, J. Pachl, T. Takahashi, C. Peckelsen, E. Cordasco, C.S. Chang, S. Oeyen, N. Aikawa, T. Maruyama, R. Schein, A.C. Kalil, M. Van Nuffelen, M. Lynn, D.P. Rossignol, J. Gogate, M.B. Roberts, J.L. Wheeler, and J.L. Vincent. 2013. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. Jama 309: 1154–1162.
Hosac, A.M., 2002. Drotrecogin alfa (activated): the first FDA-approved treatment for severe sepsis. Proceedings (Baylor University. Medical center) 15, 224-227.
Ranieri, V.M., B.T. Thompson, P.S. Barie, J.-F. Dhainaut, I.S. Douglas, S. Finfer, B. Gårdlund, J.C. Marshall, A. Rhodes, A. Artigas, D. Payen, J. Tenhunen, H.R. Al-Khalidi, V. Thompson, J. Janes, W.L. Macias, B. Vangerow, and M.D. Williams. 2012. Drotrecogin alfa (activated) in adults with septic shock. New England Journal of Medicine 366: 2055–2064.
Botero, JSH., Pérez, MCF., 2012. in: Azevedo, L. (Ed.), Sepsis—an ongoing and significant challenge. InTech, Rijeka, p. Ch. 01.
Geroulanos, S., and E.T. Douka. 2006. Historical perspective of the word “sepsis”. Intensive Care Medicine 32: 2077.
Jamme, M., F. Daviaud, J. Charpentier, N. Marin, M. Thy, Y. Hourmant, J.P. Mira, and F. Pene. 2017. Time course of septic shock in immunocompromised and nonimmunocompromised patients. Critical Care Medicine 45: 2031–2039.
Tolsma, V., C. Schwebel, E. Azoulay, M. Darmon, B. Souweine, A. Vesin, D. Goldgran-Toledano, M. Lugosi, S. Jamali, C. Cheval, C. Adrie, H. Kallel, A. Descorps-Declere, M. Garrouste-Orgeas, L. Bouadma, and J.F. Timsit. 2014. Sepsis severe or septic shock: outcome according to immune status and immunodeficiency profile. Chest 146: 1205–1213.
Man, K., V.I. Kutyavin, and A. Chawla. 2017. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metabolism 25: 11–26.
Gaber, T., C. Strehl, and F. Buttgereit. 2017. Metabolic regulation of inflammation. Nature reviews. Rheumatology 13: 267–279.
Hotamisligil, G.S. 2017. Inflammation, metaflammation and immunometabolic disorders. Nature 542: 177–185.
O'Neill, L.A., R.J. Kishton, and J. Rathmell. 2016. A guide to immunometabolism for immunologists. Nature Reviews. Immunology 16: 553–565.
Stienstra, R., R.T. Netea-Maier, N.P. Riksen, L.A.B. Joosten, and M.G. Netea. 2017. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metabolism 26: 142–156.
Evangelatos, N., Bauer, P., Reumann, M., Satyamoorthy, K., Lehrach, H., Brand, A., 2018. Metabolomics in sepsis and its impact on public health. Public health genomics.
Everts, B. 2018. Metabolomics in immunology research. Methods in molecular biology (Clifton, N.J.) 1730: 29–42.
Nasa, P., D. Juneja, and O. Singh. 2012. Severe sepsis and septic shock in the elderly: an overview. World journal of critical care medicine 1: 23–30.
Bantug, G.R., L. Galluzzi, G. Kroemer, and C. Hess. 2018. The spectrum of T cell metabolism in health and disease. Nature Reviews. Immunology 18: 19–34.
Eelen, G., P. de Zeeuw, M. Simons, and P. Carmeliet. 2015. Endothelial cell metabolism in normal and diseased vasculature. Circulation Research 116: 1231–1244.
Gleeson, L.E., and F.J. Sheedy. 2016. Metabolic reprogramming & inflammation: fuelling the host response to pathogens. Seminars in Immunology 28: 450–468.
Pircher, A., L. Treps, N. Bodrug, and P. Carmeliet. 2016. Endothelial cell metabolism: a novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis 253: 247–257.
Rohlenova, K., K. Veys, I. Miranda-Santos, K. De Bock, and P. Carmeliet. 2018. Endothelial cell metabolism in health and disease. Trends in Cell Biology 28: 224–236.
O'Neill, L.A., and E.J. Pearce. 2016. Immunometabolism governs dendritic cell and macrophage function. The Journal of Experimental Medicine 213: 15–23.
Wang, A., S.C. Huen, H.H. Luan, S. Yu, C. Zhang, J.D. Gallezot, C.J. Booth, and R. Medzhitov. 2016a. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166: 1512–1525.e1512.
Gardiner, C.M., and D.K. Finlay. 2017. What fuels natural killers? Metabolism and NK cell responses. Frontiers in Immunology 8: 367.
Mills, E.L., B. Kelly, and L.A.J. O'Neill. 2017. Mitochondria are the powerhouses of immunity. Nature Immunology 18: 488–498.
Monlun, M., C. Hyernard, P. Blanco, L. Lartigue, and B. Faustin. 2017. Mitochondria as molecular platforms integrating multiple innate immune signalings. Journal of Molecular Biology 429: 1–13.
Dale, D.C., L. Boxer, and W.C. Liles. 2008. The phagocytes: neutrophils and monocytes. Blood 112: 935–945.
Del Fresno, C., and A. Hidalgo. 2017. Neutrophils acROSs the enemy lines. Immunity 46: 335–337.
Kumar, V., and A. Sharma. 2010. Neutrophils: Cinderella of innate immune system. International Immunopharmacology 10: 1325–1334.
Mócsai, A. 2013. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. The Journal of Experimental Medicine 210: 1283–1299.
Nicolas-Avila, J.A., J.M. Adrover, and A. Hidalgo. 2017. Neutrophils in homeostasis, immunity, and cancer. Immunity 46: 15–28.
de Kleijn, S., M. Kox, I.E. Sama, J. Pillay, A. van Diepen, M.A. Huijnen, J.G. van der Hoeven, G. Ferwerda, P.W.M. Hermans, and P. Pickkers. 2012. Transcriptome kinetics of circulating neutrophils during human experimental endotoxemia. PLoS One 7: e38255.
Moulding, D.A., J.A. Quayle, C.A. Hart, and S.W. Edwards. 1998. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood 92: 2495–2502.
Shen, X.F., K. Cao, J.P. Jiang, W.X. Guan, and J.F. Du. 2017. Neutrophil dysregulation during sepsis: an overview and update. Journal of Cellular and Molecular Medicine 21: 1687–1697.
Sônego, F., F.V.S. Castanheira, R.G. Ferreira, A. Kanashiro, C.A.V.G. Leite, D.C. Nascimento, D.F. Colón, V.F. Borges, J.C. Alves-Filho, and F.Q. Cunha. 2016. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Frontiers in Immunology 7: 155.
Weinmann, P., P. Gaehtgens, and B. Walzog. 1999. Bcl-Xl- and Bax-alpha-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood 93: 3106–3115.
Jia, S.H., J. Parodo, E. Charbonney, J.L.Y. Tsang, S.Y. Jia, O.D. Rotstein, A. Kapus, and J.C. Marshall. 2014. Activated neutrophils induce epithelial cell apoptosis through oxidant-dependent tyrosine dephosphorylation of caspase-8. The American Journal of Pathology 184: 1030–1040.
Wang, J.F., J.B. Li, Y.J. Zhao, W.J. Yi, J.J. Bian, X.J. Wan, K.M. Zhu, and X.M. Deng. 2015. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: an animal study and a prospective case-control study. Anesthesiology 122: 852–863.
Arasanz, H., M. Gato-Cañas, M. Zuazo, M. Ibañez-Vea, K. Breckpot, G. Kochan, and D. Escors. 2017. PD1 signal transduction pathways in T cells. Oncotarget 8: 51936–51945.
Riley, J.L. 2009. PD-1 signaling in primary T cells. Immunological Reviews 229: 114–125.
Chemnitz, J.M., R.V. Parry, K.E. Nichols, C.H. June, and J.L. Riley. 2004. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. Journal of immunology (Baltimore, Md. : 1950) 173: 945–954.
Hui, E., J. Cheung, J. Zhu, X. Su, M.J. Taylor, H.A. Wallweber, D.K. Sasmal, J. Huang, J.M. Kim, I. Mellman, and R.D. Vale. 2017. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, N.Y.) 355: 1428–1433.
Lorenz, U. 2009. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunological Reviews 228: 342–359.
Sheppard, K.A., L.J. Fitz, J.M. Lee, C. Benander, J.A. George, J. Wooters, Y. Qiu, J.M. Jussif, L.L. Carter, C.R. Wood, and D. Chaudhary. 2004. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Letters 574: 37–41.
Patsoukis, N., L. Li, D. Sari, V. Petkova, and V.A. Boussiotis. 2013. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Molecular and Cellular Biology 33: 3091–3098.
Patsoukis, N., K. Bardhan, P. Chatterjee, D. Sari, B. Liu, L.N. Bell, E.D. Karoly, G.J. Freeman, V. Petkova, P. Seth, L. Li, and V.A. Boussiotis. 2015. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nature Communications 6: 6692.
Wong, H.R., R.J. Freishtat, M. Monaco, K. Odoms, and T.P. Shanley. 2010. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 11: 349–355.
Fox, E.D., D.S. Heffernan, W.G. Cioffi, and J.S. Reichner. 2013. Neutrophils from critically ill septic patients mediate profound loss of endothelial barrier integrity. Critical care (London, England) 17: R226.
Rimmele, T., Payen, D., Cantaluppi, V., Marshall, J., Gomez, H., Gomez, A., Murray, P., Kellum, J.A., 2016. IMMUNE CELL PHENOTYPE AND FUNCTION IN SEPSIS. Shock (Augusta, Ga.) 45, 282-291.
Hoesel, L.M., T.A. Neff, S.B. Neff, J.G. Younger, E.W. Olle, H. Gao, M.J. Pianko, K.D. Bernacki, J.V. Sarma, and P.A. Ward. 2005. Harmful and protective roles of neutrophils in sepsis. Shock (Augusta, Ga.) 24: 40–47.
Alves-Filho, J.C., F. Spiller, and F.Q. Cunha. 2010. Neutrophil paralysis in sepsis. Shock (Augusta, Ga.) 34 (Suppl 1): 15–21.
Bermejo-Martín, J.F., E. Tamayo, G. Ruiz, D. Andaluz-Ojeda, R. Herrán-Monge, A. Muriel-Bombín, M. Fe Muñoz, M. Heredia-Rodríguez, R. Citores, J.I. Gómez-Herreras, and J. Blanco. 2014. Circulating neutrophil counts and mortality in septic shock. Critical Care 18: 407–407.
Mare, T.A., D.F. Treacher, M. Shankar-Hari, R. Beale, S.M. Lewis, D.J. Chambers, and K.A. Brown. 2015. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Critical care (London, England) 19: –57.
Demaret, J., F. Venet, A. Friggeri, M.A. Cazalis, J. Plassais, L. Jallades, C. Malcus, F. Poitevin-Later, J. Textoris, A. Lepape, and G. Monneret. 2015. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. Journal of Leukocyte Biology 98: 1081–1090.
Brown, K.A., S.D. Brain, J.D. Pearson, J.D. Edgeworth, S.M. Lewis, and D.F. Treacher. 2006. Neutrophils in development of multiple organ failure in sepsis. Lancet (London, England) 368: 157–169.
Meyer-Hoffert, U., and O. Wiedow. 2011. Neutrophil serine proteases: mediators of innate immune responses. Current Opinion in Hematology 18: 19–24.
Delabranche, X., L. Stiel, F. Severac, A.C. Galoisy, L. Mauvieux, F. Zobairi, T. Lavigne, F. Toti, E. Angles-Cano, F. Meziani, and J. Boisrame-Helms. 2017. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock (Augusta, Ga.) 47: 313–317.
McDonald, B., R.P. Davis, S.J. Kim, M. Tse, C.T. Esmon, E. Kolaczkowska, and C.N. Jenne. 2017. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129: 1357–1367.
Evans, W.H., Karnovsky, M.L., 1962. The biochemical basis of phagocytosis. IV. Some aspects of carbohydrate metabolism during phagocytosis. Biochemistry 1, 159–166.
Lodhi, I.J., X. Wei, L. Yin, C. Feng, S. Adak, G. Abou-Ezzi, F.F. Hsu, D.C. Link, and C.F. Semenkovich. 2015. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metabolism 21: 51–64.
Sbarra, A.J., and M.L. Karnovsky. 1959. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. The Journal of biological chemistry 234: 1355–1362.
Biswas, S.K. 2015. Metabolic reprogramming of immune cells in cancer progression. Immunity 43: 435–449.
Borregaard, N., and T. Herlin. 1982. Energy metabolism of human neutrophils during phagocytosis. The Journal of Clinical Investigation 70: 550–557.
Jun, H.S., D.A. Weinstein, Y.M. Lee, B.C. Mansfield, and J.Y. Chou. 2014. Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood 123: 2843–2853.
Guthrie, L.A., L.C. McPhail, P.M. Henson, and R.B. Johnston Jr. 1984. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. The Journal of experimental medicine 160: 1656–1671.
Winterbourn, C.C., A.J. Kettle, and M.B. Hampton. 2016. Reactive oxygen species and neutrophil function. Annual Review of Biochemistry 85: 765–792.
Azevedo, E.P., N.C. Rochael, A.B. Guimaraes-Costa, T.S. de Souza-Vieira, J. Ganilho, E.M. Saraiva, F.L. Palhano, and D. Foguel. 2015. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. The Journal of Biological Chemistry 290: 22174–22183.
Park, D.W., and J.W. Zmijewski. 2017. Mitochondrial dysfunction and immune cell metabolism in sepsis. Infection & chemotherapy 49: 10–21.
Rodriguez-Espinosa, O., O. Rojas-Espinosa, M.M. Moreno-Altamirano, E.O. Lopez-Villegas, and F.J. Sanchez-Garcia. 2015. Metabolic requirements for neutrophil extracellular traps formation. Immunology 145: 213–224.
Maianski, N.A., J. Geissler, S.M. Srinivasula, E.S. Alnemri, D. Roos, and T.W. Kuijpers. 2004. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death and Differentiation 11: 143–153.
Pearce, Erika L., and Edward J. Pearce. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity 38: 633–643.
Fossati, G., D.A. Moulding, D.G. Spiller, R.J. Moots, M.R. White, and S.W. Edwards. 2003. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. Journal of immunology (Baltimore, Md. : 1950) 170: 1964–1972.
van Raam, B.J., W. Sluiter, E. de Wit, D. Roos, A.J. Verhoeven, and T.W. Kuijpers. 2008. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One 3: e2013.
Remijsen, Q., T.W. Kuijpers, E. Wirawan, S. Lippens, P. Vandenabeele, and T. Vanden Berghe. 2011. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death and Differentiation 18: 581.
Lu, H., R.A. Forbes, and A. Verma. 2002. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. The Journal of Biological Chemistry 277: 23111–23115.
Sun, Q., X. Chen, J. Ma, H. Peng, F. Wang, X. Zha, Y. Wang, Y. Jing, H. Yang, R. Chen, L. Chang, Y. Zhang, J. Goto, H. Onda, T. Chen, M.R. Wang, Y. Lu, H. You, D. Kwiatkowski, and H. Zhang. 2011. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proceedings of the National Academy of Sciences of the United States of America 108: 4129–4134.
Cramer, T., Y. Yamanishi, B.E. Clausen, I. Forster, R. Pawlinski, N. Mackman, V.H. Haase, R. Jaenisch, M. Corr, V. Nizet, G.S. Firestein, H.P. Gerber, N. Ferrara, and R.S. Johnson. 2003. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657.
Halligan, D.N., S.J. Murphy, and C.T. Taylor. 2016. The hypoxia-inducible factor (HIF) couples immunity with metabolism. Seminars in Immunology 28: 469–477.
Semenza, G.L., P.H. Roth, H.M. Fang, and G.L. Wang. 1994. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. The Journal of Biological Chemistry 269: 23757–23763.
Peyssonnaux, C., V. Datta, T. Cramer, A. Doedens, E.A. Theodorakis, R.L. Gallo, N. Hurtado-Ziola, V. Nizet, and R.S. Johnson. 2005. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. The Journal of Clinical Investigation 115: 1806–1815.
Cheng, S.C., J. Quintin, R.A. Cramer, K.M. Shepardson, S. Saeed, V. Kumar, E.J. Giamarellos-Bourboulis, J.H. Martens, N.A. Rao, A. Aghajanirefah, G.R. Manjeri, Y. Li, D.C. Ifrim, R.J. Arts, B.M. van der Veer, P.M. Deen, C. Logie, L.A. O'Neill, P. Willems, F.L. van de Veerdonk, J.W. van der Meer, A. Ng, L.A. Joosten, C. Wijmenga, H.G. Stunnenberg, R.J. Xavier, and M.G. Netea. 2014. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (New York, N.Y.) 345: 1250684.
Delano, M.J., and P.A. Ward. 2016. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunological Reviews 274: 330–353.
Howell, J.J., and B.D. Manning. 2011. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends in Endocrinology and Metabolism: TEM 22: 94–102.
Krawczyk, C.M., T. Holowka, J. Sun, J. Blagih, E. Amiel, R.J. DeBerardinis, J.R. Cross, E. Jung, C.B. Thompson, R.G. Jones, and E.J. Pearce. 2010. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115: 4742–4749.
Liu, L., S. Das, W. Losert, and C.A. Parent. 2010. mTORC2 REGULATES NEUTROPHIL CHEMOTAXIS IN A cAMP- AND RhoA-DEPENDENT FASHION. Developmental Cell 19: 845–857.
Itakura, A., and O.J.T. McCarty. 2013. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. American Journal of Physiology - Cell Physiology 305: C348–C354.
McInturff, A.M., M.J. Cody, E.A. Elliott, J.W. Glenn, J.W. Rowley, M.T. Rondina, and C.C. Yost. 2012. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 120: 3118–3125.
Chen, F., A. Cao, S. Yao, H.L. Evans-Marin, H. Liu, W. Wu, E.D. Carlsen, S.M. Dann, L. Soong, J. Sun, Q. Zhao, and Y. Cong. 2016. mTOR mediates IL-23 induction of neutrophil IL-17 and IL-22 production. Journal of immunology (Baltimore, Md. : 1950) 196: 4390–4399.
Kumar, V. 2013. Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signalling 9: 145–165.
Kwak, Y., H.E. Kim, and S.G. Park. 2015. Insights into myeloid-derived suppressor cells in inflammatory diseases. Archivum Immunologiae et Therapiae Experimentalis (Warsz) 63: 269–285.
Ost, M., Singh, A., Peschel, A., Mehling, R., Rieber, N., Hartl, D., 2016. Myeloid-derived suppressor cells in bacterial infections. Frontiers in Cellular and Infection Microbiology 6.
Ostrand-Rosenberg, S., and P. Sinha. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. The Journal of Immunology 182: 4499–4506.
Gabrilovich, D.I., and S. Nagaraj. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology 9: 162–174.
Brudecki, L., D.A. Ferguson, C.E. McCall, and M. El Gazzar. 2012. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infection and Immunity 80: 2026–2034.
Delano, M.J., P.O. Scumpia, J.S. Weinstein, D. Coco, S. Nagaraj, K.M. Kelly-Scumpia, K.A. O'Malley, J.L. Wynn, S. Antonenko, S.Z. Al-Quran, R. Swan, C.S. Chung, M.A. Atkinson, R. Ramphal, D.I. Gabrilovich, W.H. Reeves, A. Ayala, J. Phillips, D. Laface, P.G. Heyworth, M. Clare-Salzler, and L.L. Moldawer. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of Experimental Medicine 204: 1463–1474.
Derive, M., Y. Bouazza, C. Alauzet, and S. Gibot. 2012. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Medicine 38: 1040–1049.
Cuenca, A.G., and L.L. Moldawer. 2012. Myeloid-derived suppressor cells in sepsis: friend or foe? Intensive Care Medicine 38: 928–930.
Lai, D., C. Qin, and Q. Shu. 2014. Myeloid-derived suppressor cells in sepsis. BioMed Research International 2014: 8.
Uhel, F., I. Azzaoui, M. Gregoire, C. Pangault, J. Dulong, J.M. Tadie, A. Gacouin, C. Camus, L. Cynober, T. Fest, Y. Le Tulzo, M. Roussel, and K. Tarte. 2017. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis. American Journal of Respiratory and Critical Care Medicine 196: 315–327.
Goh, C., S. Narayanan, and Y.S. Hahn. 2013. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunological Reviews 255: 210–221.
Haile, L.A., R. von Wasielewski, J. Gamrekelashvili, C. Kruger, O. Bachmann, A.M. Westendorf, J. Buer, R. Liblau, M.P. Manns, F. Korangy, and T.F. Greten. 2008. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135: 871–881 881.e871-875.
Kim, Y.-J., S.-Y. Chang, and H.-J. Ko. 2015. Myeloid-derived suppressor cells in inflammatory bowel disease. Intestinal Research 13: 105–111.
Qin, A., W. Cai, T. Pan, K. Wu, Q. Yang, N. Wang, Y. Liu, D. Yan, F. Hu, P. Guo, X. Chen, L. Chen, H. Zhang, X. Tang, and J. Zhou. 2013. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. Journal of Virology 87: 1477–1490.
Tacke, R.S., H.C. Lee, C. Goh, J. Courtney, S.J. Polyak, H.R. Rosen, and Y.S. Hahn. 2012. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology (Baltimore, Md.) 55: 343–353.
Bunt, S.K., P. Sinha, V.K. Clements, J. Leips, and S. Ostrand-Rosenberg. 2006. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. Journal of immunology (Baltimore, Md. : 1950) 176: 284–290.
Porta, C., Marino, A., Consonni, F.M., Bleve, A., Mola, S., Storto, M., Riboldi, E., Sica, A., 2018. Metabolic influence on the differentiation of suppressive myeloid cells in cancer. Carcinogenesis, bgy088-bgy088.
Tu, S., G. Bhagat, G. Cui, S. Takaishi, E.A. Kurt-Jones, B. Rickman, K.S. Betz, M. Penz-Oesterreicher, O. Bjorkdahl, J.G. Fox, and T.C. Wang. 2008. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14: 408–419.
Hossain, F., A.A. Al-Khami, D. Wyczechowska, C. Hernandez, L. Zheng, K. Reiss, L.D. Valle, J. Trillo-Tinoco, T. Maj, W. Zou, P.C. Rodriguez, and A.C. Ochoa. 2015. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunology Research 3: 1236–1247.
Gabrilovich, D.I., S. Ostrand-Rosenberg, and V. Bronte. 2012. Coordinated regulation of myeloid cells by tumours. Nature Reviews. Immunology 12: 253–268.
Cai, T.-T., S.-B. Ye, Y.-N. Liu, J. He, Q.-Y. Chen, H.-Q. Mai, C.-X. Zhang, J. Cui, X.-S. Zhang, P. Busson, Y.-X. Zeng, and J. Li. 2017. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathogens 13: e1006503.
Hammami, I., J. Chen, F. Murschel, V. Bronte, G. De Crescenzo, and M. Jolicoeur. 2012. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biology 13: 18.
Corzo, C.A., T. Condamine, L. Lu, M.J. Cotter, J.I. Youn, P. Cheng, H.I. Cho, E. Celis, D.G. Quiceno, T. Padhya, T.V. McCaffrey, J.C. McCaffrey, and D.I. Gabrilovich. 2010. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of Experimental Medicine 207: 2439–2453.
Noman, M.Z., G. Desantis, B. Janji, M. Hasmim, S. Karray, P. Dessen, V. Bronte, and S. Chouaib. 2014. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. The Journal of Experimental Medicine 211: 781–790.
Marcu, R., Y.J. Choi, J. Xue, C.L. Fortin, Y. Wang, R.J. Nagao, J. Xu, J.W. MacDonald, T.K. Bammler, C.E. Murry, K. Muczynski, K.R. Stevens, J. Himmelfarb, S.M. Schwartz, and Y. Zheng. 2018. Human organ-specific endothelial cell heterogeneity. iScience 4: 20–35.
Ivanov, A.N., I.A. Norkin, D.M. Puchin'ian, V. Shirokov, and O. Zhdanova. 2014. Endothelial cell adhesion molecules. Uspekhi Fiziologicheskikh Nauk 45: 34–49.
Ye, X., J. Ding, X. Zhou, G. Chen, and S.F. Liu. 2008. Divergent roles of endothelial NF-κB in multiple organ injury and bacterial clearance in mouse models of sepsis. The Journal of Experimental Medicine 205: 1303–1315.
Kim, B., C. Jang, H. Dharaneeswaran, J. Li, M. Bhide, S. Yang, K. Li, and Z. Arany. 2018. Endothelial pyruvate kinase M2 maintains vascular integrity. The Journal of Clinical Investigation 128: 4543–4556.
Parikh, S.M. 2013. Dysregulation of the angiopoietin–Tie-2 axis in sepsis and ARDS. Virulence 4: 517–524.
Parikh, S.M. 2017. The angiopoietin-Tie2 signaling axis in systemic inflammation. Journal of the American Society of Nephrology 28: 1973–1982.
Scholz, A., K.H. Plate, and Y. Reiss. 2015. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Annals of the New York Academy of Sciences 1347: 45–51.
De Bock, K., M. Georgiadou, S. Schoors, A. Kuchnio, B.W. Wong, A.R. Cantelmo, A. Quaegebeur, B. Ghesquiere, S. Cauwenberghs, G. Eelen, L.K. Phng, I. Betz, B. Tembuyser, K. Brepoels, J. Welti, I. Geudens, I. Segura, B. Cruys, F. Bifari, I. Decimo, R. Blanco, S. Wyns, J. Vangindertael, S. Rocha, R.T. Collins, S. Munck, D. Daelemans, H. Imamura, R. Devlieger, M. Rider, P.P. Van Veldhoven, F. Schuit, R. Bartrons, J. Hofkens, P. Fraisl, S. Telang, R.J. Deberardinis, L. Schoonjans, S. Vinckier, J. Chesney, H. Gerhardt, M. Dewerchin, and P. Carmeliet. 2013. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154: 651–663.
DeBerardinis, R.J., J.J. Lum, G. Hatzivassiliou, and C.B. Thompson. 2008. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism 7: 11–20.
Pavlova, N.N., and C.B. Thompson. 2016. The emerging hallmarks of cancer metabolism. Cell Metabolism 23: 27–47.
Wu, G., T.E. Haynes, H. Li, and C.J. Meininger. 2000. Glutamine metabolism in endothelial cells: ornithine synthesis from glutamine via pyrroline-5-carboxylate synthase. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology 126: 115–123.
Leighton, B., R. Curi, A. Hussein, and E.A. Newsholme. 1987. Maximum activities of some key enzymes of glycolysis, glutaminolysis, Krebs cycle and fatty acid utilization in bovine pulmonary endothelial cells. FEBS Letters 225: 93–96.
Unterluggauer, H., S. Mazurek, B. Lener, E. Hutter, E. Eigenbrodt, W. Zwerschke, and P. Jansen-Durr. 2008. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology 9: 247–259.
Eelen, G., P.D. Zeeuw, L. Treps, U. Harjes, B.W. Wong, and P. Carmeliet. 2018. Endothelial cell metabolism. Physiological Reviews 98: 3–58.
Schoors, S., U. Bruning, R. Missiaen, K.C. Queiroz, G. Borgers, I. Elia, A. Zecchin, A.R. Cantelmo, S. Christen, J. Goveia, W. Heggermont, L. Godde, S. Vinckier, P.P. Van Veldhoven, G. Eelen, L. Schoonjans, H. Gerhardt, M. Dewerchin, M. Baes, K. De Bock, B. Ghesquiere, S.Y. Lunt, S.M. Fendt, and P. Carmeliet. 2015. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520: 192–197.
Wong, B.W., E. Marsch, L. Treps, M. Baes, and P. Carmeliet. 2017. Endothelial cell metabolism in health and disease: impact of hypoxia. The EMBO Journal. 36(15): 2187–2203.
Karlsson, S., V. Pettila, J. Tenhunen, V. Lund, S. Hovilehto, and E. Ruokonen. 2008. Vascular endothelial growth factor in severe sepsis and septic shock. Anesthesia and Analgesia 106: 1820–1826.
Smadja, D.M., D. Borgel, J.L. Diehl, and P. Gaussem. 2012. Vascular endothelial growth factor, as compared with placental growth factor, is increased in severe sepsis but not in organ failure. Journal of Thrombosis and Haemostasis 10: 974–976.
van der Flier, M., H.J. van Leeuwen, K.P. van Kessel, J.L. Kimpen, A.I. Hoepelman, and S.P. Geelen. 2005. Plasma vascular endothelial growth factor in severe sepsis. Shock (Augusta, Ga.) 23: 35–38.
El-Akabawy, H., M.A. Hamela, A. Gaber, and A. Abozekry. 2016. Prognostic value of vascular endothelial growth factor in sepsis syndrome. The Egyptian Journal of Critical Care Medicine 4: 119–126.
Diskin, C., and E.M. Palsson-McDermott. 2018. Metabolic modulation in macrophage effector function. Frontiers in Immunology 9: 270.
Koppenol, W.H., P.L. Bounds, and C.V. Dang. 2011. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature Reviews. Cancer 11: 325–337.
Fukuzumi, M., H. Shinomiya, Y. Shimizu, K. Ohishi, and S. Utsumi. 1996. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infection and Immunity 64: 108–112.
Tur, J., T. Vico, J. Lloberas, A. Zorzano, and A. Celada. 2017. Macrophages and mitochondria: a critical interplay between metabolism, signaling, and the functional activity. Advances in Immunology 133: 1–36.
Rodríguez-Prados, J.-C., P.G. Través, J. Cuenca, D. Rico, J. Aragonés, P. Martín-Sanz, M. Cascante, and L. Boscá. 2010. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. The Journal of Immunology 185: 605–614.
Theodorakis, E., Diamantaki, E., Tsatsanis, C., Georgopoulos, D., Vaporidi, K., 2015. Macrophage phenotype in sepsis immunosuppression. Critical Care 19, P44-P44.
Watanabe, N., Y. Suzuki, S. Inokuchi, and S. Inoue. 2016. Sepsis induces incomplete M2 phenotype polarization in peritoneal exudate cells in mice. Journal of Intensive Care 4: 6.
Kumar, V. 2018b. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. International Immunopharmacology 58: 173–185.
Hotchkiss, R.S., P.E. Swanson, B.D. Freeman, K.W. Tinsley, J.P. Cobb, G.M. Matuschak, T.G. Buchman, and I.E. Karl. 1999. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine 27: 1230–1251.
Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, R.E. Schmieg Jr., J.J. Hui, K.C. Chang, D.F. Osborne, B.D. Freeman, J.P. Cobb, T.G. Buchman, and I.E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of immunology (Baltimore, Md. : 1950) 166: 6952–6963.
Inoue, S., K. Suzuki-Utsunomiya, Y. Okada, T. Taira, Y. Iida, N. Miura, T. Tsuji, T. Yamagiwa, S. Morita, T. Chiba, T. Sato, and S. Inokuchi. 2013. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Critical Care Medicine 41: 810–819.
Wang, S.D., K.J. Huang, Y.S. Lin, and H.Y. Lei. 1994. Sepsis-induced apoptosis of the thymocytes in mice. Journal of immunology (Baltimore, Md. : 1950) 152: 5014–5021.
Chapman, N.M., S. Shrestha, and H. Chi. 2017. Metabolism in immune cell differentiation and function. Advances in Experimental Medicine and Biology 1011: 1–85.
MacIver, N.J., R.D. Michalek, and J.C. Rathmell. 2013. Metabolic regulation of T lymphocytes. Annual Review of Immunology 31: 259–283.
Venet, F., and G. Monneret. 2018. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nature Reviews. Nephrology 14: 121–137.
Andreu-Ballester, J.C., C. Cuellar, C. Garcia-Ballesteros, J. Perez-Griera, V. Amigo, A. Peiro-Gomez, C. Penarroja-Otero, F. Ballester, J. Mayans, and C. Tormo-Calandin. 2014. Deficit of interleukin 7 in septic patients. International Immunopharmacology 23: 73–76.
Bauer, M., E.J. Giamarellos-Bourboulis, A. Kortgen, E. Moller, K. Felsmann, J.M. Cavaillon, O. Guntinas-Lichius, O. Rutschmann, A. Ruryk, M. Kohl, B. Wlotzka, S. Russwurm, J.C. Marshall, and K. Reinhart. 2016. A transcriptomic biomarker to quantify systemic inflammation in sepsis—a prospective multicenter phase II diagnostic study. EBioMedicine 6: 114–125.
Wofford, J.A., H.L. Wieman, S.R. Jacobs, Y. Zhao, and J.C. Rathmell. 2008. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111: 2101–2111.
Beier, U.H., A. Angelin, T. Akimova, L. Wang, Y. Liu, H. Xiao, M.A. Koike, S.A. Hancock, T.R. Bhatti, R. Han, J. Jiao, S.C. Veasey, C.A. Sims, J.A. Baur, D.C. Wallace, and W.W. Hancock. 2015. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29: 2315–2326.
Michalek, R.D., V.A. Gerriets, S.R. Jacobs, A.N. Macintyre, N.J. MacIver, E.F. Mason, S.A. Sullivan, A.G. Nichols, and J.C. Rathmell. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology (Baltimore, Md. : 1950) 186: 3299–3303.
Newton, R., B. Priyadharshini, and L.A. Turka. 2016. Immunometabolism of regulatory T cells. Nature Immunology 17: 618–625.
Zeng, H., K. Yang, C. Cloer, G. Neale, P. Vogel, and H. Chi. 2013. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499: 485–490.
Kumar, V. 2018a. T cells and their immunometabolism: a novel way to understanding sepsis immunopathogenesis and future therapeutics. European Journal of Cell Biology.
Nalos, M., G. Parnell, R. Robergs, D. Booth, A.S. McLean, and B.M. Tang. 2016. Transcriptional reprogramming of metabolic pathways in critically ill patients. Intensive care medicine experimental 4: 21.
Dugnani, E., V. Pasquale, C. Bordignon, A. Canu, L. Piemonti, and P. Monti. 2017. Integrating T cell metabolism in cancer immunotherapy. Cancer Letters 411: 12–18.
Mockler, M.B., M.J. Conroy, and J. Lysaght. 2014. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Frontiers in Oncology 4: 107.
Hotchkiss, R.S., and L.L. Moldawer. 2014. Parallels between cancer and infectious disease. The New England Journal of Medicine 371: 380–383.
Hotchkiss, R.S., Moldawer, L.L., Opal, S.M., Reinhart, K., Turnbull, I.R., Vincent, J.L., 2016. Sepsis and septic shock. Nature reviews. Disease primers 2, 16045.
Zhang, Z., Deng, W., Kang, R., Xie, M., Billiar, T., Wang, H., Cao, L., Tang, D., 2016. Plumbagin protects mice from lethal sepsis by modulating immunometabolism upstream of PKM2. Molecular medicine (Cambridge, Mass.).
O'Neill, L.A., and D.G. Hardie. 2013. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493: 346–355.
Sag, D., D. Carling, R.D. Stout, and J. Suttles. 2008. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. Journal of immunology (Baltimore, Md. : 1950) 181: 8633–8641.
Zhu, Y.P., J.R. Brown, D. Sag, L. Zhang, and J. Suttles. 2015. Adenosine 5′-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. Journal of immunology (Baltimore, Md. : 1950) 194: 584–594.
Escobar, D.A., A.M. Botero-Quintero, B.C. Kautza, J. Luciano, P. Loughran, S. Darwiche, M.R. Rosengart, B.S. Zuckerbraun, and H. Gomez. 2015. AMPK activation protects against sepsis-induced organ injury and inflammation. The Journal of Surgical Research 194: 262–272.
Li, Y., N. Nourbakhsh, E. Hall, M. Hepokoski, H. Pham, J. Thomas, and P. Singh. 2016. Protective role of AMPK in sepsis associated AKI. The FASEB Journal 30: 1217.1218.
Castanares-Zapatero, D., M. Overtus, D. Communi, M. Horckmans, L. Bertrand, C. Oury, C. Lecut, P. Laterre, S. De man, C. Sommereyns, S. Horman, and C. Beauloye. 2012. AMP-activated protein kinase controls liposaccharide-induced hyperpermeability. Critical Care 16: P17.
Huang, J., K. Liu, S. Zhu, M. Xie, R. Kang, L. Cao, and D. Tang. 2017. AMPK regulates immunometabolism in sepsis. Brain, Behavior, and Immunity.
Taylor, C.T., and S.P. Colgan. 2017. Regulation of immunity and inflammation by hypoxia in immunological niches. Nature Reviews. Immunology 17: 774–785.
Liu, Z., N. Bone, S. Jiang, D.W. Park, J.M. Tadie, J. Deshane, C.A. Rodriguez, J.F. Pittet, E. Abraham, and J.W. Zmijewski. 2015. AMP-activated protein kinase and glycogen synthase kinase 3beta modulate the severity of sepsis-induced lung injury. Molecular medicine 21(1): 937–950.
Cheng, S.C., B.P. Scicluna, R.J. Arts, M.S. Gresnigt, E. Lachmandas, E.J. Giamarellos-Bourboulis, M. Kox, G.R. Manjeri, J.A. Wagenaars, O.L. Cremer, J. Leentjens, A.J. van der Meer, F.L. van de Veerdonk, M.J. Bonten, M.J. Schultz, P.H. Willems, P. Pickkers, L.A. Joosten, T. van der Poll, and M.G. Netea. 2016. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nature Immunology 17: 406–413.
Jha, A.K., S.C. Huang, A. Sergushichev, V. Lampropoulou, Y. Ivanova, E. Loginicheva, K. Chmielewski, K.M. Stewart, J. Ashall, B. Everts, E.J. Pearce, E.M. Driggers, and M.N. Artyomov. 2015. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42: 419–430.
Van den Bossche, J., L.A. O'Neill, and D. Menon. 2017. Macrophage immunometabolism: where are we (going)? Trends in Immunology 38: 395–406.
Mills, E.L., B. Kelly, A. Logan, A.S.H. Costa, M. Varma, C.E. Bryant, P. Tourlomousis, J.H.M. Däbritz, E. Gottlieb, I. Latorre, S.C. Corr, G. McManus, D. Ryan, H.T. Jacobs, M. Szibor, R.J. Xavier, T. Braun, C. Frezza, M.P. Murphy, and L.A. O’Neill. 2016. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167: 457–470.e413.
Patil, N.K., J.K. Bohannon, and E.R. Sherwood. 2016. Immunotherapy: a promising approach to reverse sepsis-induced immunosuppression. Pharmacological Research 111: 688–702.
Shindo, Y., A.G. Fuchs, C.G. Davis, T. Eitas, J. Unsinger, C.D. Burnham, J.M. Green, M. Morre, G.V. Bochicchio, and R.S. Hotchkiss. 2017. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. Journal of Leukocyte Biology 101: 543–554.
Venet, F., Demaret, J., Blaise, B.J., Rouget, C., Girardot, T., Idealisoa, E., Rimmele, T., Mallet, F., Lepape, A., Textoris, J., Monneret, G., 2017. IL-7 restores T lymphocyte immunometabolic failure in septic shock patients through mTOR activation. Journal of immunology (Baltimore, Md. : 1950) 199, 1606-1615.
Venet, F., Monneret, G., 2017. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nature reviews. Nephrology.
Venet, F., T. Rimmele, and G. Monneret. 2018. Management of sepsis-induced immunosuppression. Critical Care Clinics 34: 97–106.
Francois, B., Jeannet, R., Daix, T., Walton, A.H., Shotwell, M.S., Unsinger, J., Monneret, G., Rimmele, T., Blood, T., Morre, M., Gregoire, A., Mayo, G.A., Blood, J., Durum, S.K., Sherwood, E.R., Hotchkiss, R.S., 2018. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI insight 3.
Angelin, A., Gil-de-Gomez, L., Dahiya, S., Jiao, J., Guo, L., Levine, M.H., Wang, Z., Quinn, W.J., 3rd, Kopinski, P.K., Wang, L., Akimova, T., Liu, Y., Bhatti, T.R., Han, R., Laskin, B.L., Baur, J.A., Blair, I.A., Wallace, D.C., Hancock, W.W., Beier, U.H., 2017. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell metabolism 25, 1282-1293.e1287.
Grzes, K.M., C.S. Field, and E.J. Pearce. 2017. Treg cells survive and thrive in inhospitable environments. Cell Metabolism 25: 1213–1215.
Cao, C., T. Ma, Y.F. Chai, and S.T. Shou. 2015. The role of regulatory T cells in immune dysfunction during sepsis. World Journal of Emergency Medicine 6: 5–9.
Jiang, L.-N., Y.-M. Yao, and Z.-Y. Sheng. 2012. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. Journal of Interferon & Cytokine Research 32: 341–349.
Mannick, J.B., M. Morris, H.-U.P. Hockey, G. Roma, M. Beibel, K. Kulmatycki, M. Watkins, T. Shavlakadze, W. Zhou, D. Quinn, D.J. Glass, and L.B. Klickstein. 2018. TORC1 inhibition enhances immune function and reduces infections in the elderly. Science Translational Medicine 10.
Cantey, J.B., and S.D. Baird. 2017. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics 140.
Martín, S., A. Pérez, and C. Aldecoa. 2017. Sepsis and immunosenescence in the elderly patient: a review. Frontiers in Medicine 4: 20.
Mayr, F.B., S. Yende, W.T. Linde-Zwirble, O.M. Peck-Palmer, A.E. Barnato, L.A. Weissfeld, and D.C. Angus. 2010. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. Jama 303: 2495–2503.
Rowe, T.A., and J.M. McKoy. 2017. Sepsis in older adults. Infectious Disease Clinics of North America 31: 731–742.
Vardi, M., N.O. Ghanem-Zoubi, H. Bitterman, N. Abo-Helo, V. Yurin, G. Weber, and A. Laor. 2013. Sepsis in nonagenarians admitted to internal medicine departments: a comparative study of outcomes. QJM : Monthly Journal of the Association of Physicians 106: 261–266.
Prescott, H.C., and D.C. Angus. 2018. Enhancing recovery from sepsis: a review. Jama 319: 62–75.
Allahyari, H., S. Heidari, M. Ghamgosha, P. Saffarian, and J. Amani. 2017. Immunotoxin: a new tool for cancer therapy. Tumor Biology 39: 1010428317692226.
Fuchs, H., A. Weng, and R. Gilabert-Oriol. 2016. Augmenting the efficacy of immunotoxins and other targeted protein toxins by endosomal escape enhancers. Toxins 8: 200.
Markwart, R., S.A. Condotta, R.P. Requardt, F. Borken, K. Schubert, C. Weigel, M. Bauer, T.S. Griffith, M. Forster, F.M. Brunkhorst, V.P. Badovinac, and I. Rubio. 2014. Immunosuppression after sepsis: systemic inflammation and sepsis induce a loss of naive T-cells but no enduring cell-autonomous defects in T-cell function. PLoS One 9: e115094.
Author information
Authors and Affiliations
Contributions
The corresponding author generated the idea, gathered the cited literature, and wrote the article.
Corresponding author
Ethics declarations
Competing Interest
The author declares that he does not have any competing interest.
Rights and permissions
About this article
Cite this article
Kumar, V. Immunometabolism: Another Road to Sepsis and Its Therapeutic Targeting. Inflammation 42, 765–788 (2019). https://doi.org/10.1007/s10753-018-0939-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0939-8