Abstract
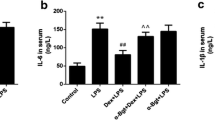
Previous studies have shown that dexmedetomidine exerted anti-inflammatory effect on several animal models with inflammation, but the mechanism is not clear. This study intends to elucidate the anti-inflammatory mechanism of dexmedetomidine through the cholinergic anti-inflammatory pathway. To investigate this therapeutic potential of dexmedetomidine, a murine model of endotoxemia was established induced by lipopolysaccharide (LPS). Animals were assigned to one of four protocols. Protocol one: animals were randomly assigned to control group, dexmedetomidine group, and sterile saline group (n = 20 each), and these animals were used for survival analysis. The survival rate was assessed up to 120 h after endotoxin injection. Protocol two: animals were randomly assigned to one of four groups (n = 16 each): group 1 (group Saline), treated with sterile saline 15 min prior to endotoxin treatment (10 mg kg−1 over 2 min); group 2 (group Dex), treated with dexmedetomidine 15 min prior to endotoxin treatment; group 3 (group αBGT + Dex), treated with alpha-7 nicotinic acetylcholine receptors (α7nAChR) antagonist alpha-bungarotoxin (αBGT, 1 μg/kg) 15 min prior to dexmedetomidine treatment; group 4 (group saline + Dex), treated with equivalent sterile saline 15 min prior to dexmedetomidine treatment. Protocol three: animals were randomly assigned to one of two groups (n = 16 each): vagotomy group (group VNX + Dex), right cervical vagus nerve was exposed and transected; sham-operated group (group SHAM + Dex), the cervical vagus nerve was visualized, but was neither isolated from the surrounding tissues nor transected. Protocol four: animals were treated with dexmedetomidine (40 μg/kg) and sterile saline to observe the discharge activity of cervical vagus nerves by using BL-420F data acquisition and analysis system (n = 16 each). In the survival analysis groups, the survival rate of dexmedetomidine group was significantly higher than that of the endotoxemia group (65 versus 25 %, P < 0.01). Preemptive administration of dexmedetomidine significantly attenuated the cytokine response after lipopolysaccharide (LPS) induced endotoxemia (TNF-alpha, IL-1beta, IL-6, P < 0.01, respectively). However, preemptive administration of dexmedetomidine failed to suppress cytokine response in α-bungarotoxin group and vagotomy group (TNF-alpha, IL-1beta, IL-6, P > 0.05, respectively). Furthermore, preemptive administration of dexmedetomidine significantly increased the discharge frequency of cervical vagus nerves in comparison with sterile saline treatment (P < 0.01).Our results demonstrate that the preemptive administration of dexmedetomidine increases the activity of cervical vagus nerve and have the ability to successfully improve survival in experimental endotoxemia by inhibiting the inflammatory cytokines release. However, administration of dexmedetomidine to vagotomy or α7 nAChR antagonist pretreatment mice failed to suppress TNF levels, indicating that the vagus nerve and α7nAChR-mediated cholinergic anti-inflammatory pathway is required for the anti-inflammatory effect of dexmedetomidine. These findings show that central alpha-2 agonist dexmedetomidine suppresses systemic inflammation through vagal- and α7nAChR-dependent mechanism.




Similar content being viewed by others
References
Casey, L.C., R.A. Balk, and R.C. Bone. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Annals of Internal Medicine 119: 771–778.
Damas, P., A. Reuter, P. Gysen, J. Demonty, M. Lamy, and P. Franchimont. 1989. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Critical Care Medicine 17: 975–978.
Wakabayashi, G., J.A. Gelfand, W.K. Jung, R.J. Connolly, J.F. Burke, and C.A. Dinarello. 1991. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. Journal of Clinical Investigation 87: 1925–1935.
Nathan, C. 2002. Points of control in inflammation. Nature 420: 846–852.
Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkina, L.R. Watkins, H. Wang, N. Abumrad, J.W. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462.
Tracey, K.J. 2002. The inflammatory reflex. Nature 420: 853–859.
Taniguchi, T., K. Yamamoto, N. Ohmoto, K. Ohta, and T. Kobayashi. 2000. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Critical Care Medicine 28: 1101–1106.
Taniguchi, T., and K. Yamamoto. 2005. Anti-inflammatory effects of intravenous anesthetics on endotoxemia. Mini Reviews in Medicinal Chemistry 5: 241–245.
Hsu, Y.W., L.I. Cortinez, K.M. Robertson, J.C. Keifer, S.T. Sum-Ping, E.W. Moretti, C.C. Young, D.R. Wright, D.B. Macleod, and J. Somma. 2004. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 101: 1066–1076.
Pandharipande, P.P., B.T. Pun, D.L. Herr, M. Maze, T.D. Girard, R.R. Miller, A.K. Shintani, J.L. Thompson, J.C. Jackson, S.A. Deppen, et al. 2007. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 298: 2644–2653.
Hofer, S., J. Steppan, T. Wagner, B. Funke, C. Lichtenstern, E. Martin, B.M. Graf, A. Bierhaus, and M.A. Weigand. 2009. Central sympatholytics prolong survival in experimental sepsis. Critical Care 13: R11.
Taniguchi, T., A. Kurita, K. Kobayashi, K. Yamamoto, and H. Inaba. 2008. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. Journal of Anesthesia 22: 221–228.
Gu, J., J. Chen, P. Xia, G. Tao, H. Zhao, and D. Ma. 2011. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiologica Scandinavica 55: 1272–1278.
Gu, J., P. Sun, H. Zhao, H.R. Watts, R.D. Sanders, N. Terrando, P. Xia, M. Maze, and D. Ma. 2011. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Critical Care 15: R153.
Sica, D.A. 2007. Centrally acting antihypertensive agents: an update. Journal of Clinical Hypertension (Greenwich, Conn.) 9: 399–405.
Rittirsch, D., M.A. Flierl, and P.A. Ward. 2008. Harmful molecular mechanisms in sepsis. Nature Reviews Immunology 8: 776–787.
Taniguchi, T., Y. Kidani, H. Kanakura, Y. Takemoto, and K. Yamamoto. 2004. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Critical Care Medicine 32: 1322–1326.
Seok, J., H.S. Warren, A.G. Cuenca, M.N. Mindrinos, H.V. Baker, W. Xu, D.R. Richards, G.P. McDonald-Smith, H. Gao, L. Hennessy, et al. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America 110: 3507–3512.
Rosas-Ballina, M., and K.J. Tracey. 2009. Cholinergic control of inflammation. Journal of Internal Medicine 265: 663–679.
van Westerloo, D.J., I.A. Giebelen, S. Florquin, J. Daalhuisen, M.J. Bruno, A.F. de Vos, K.J. Tracey, and T. van der Poll. 2005. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. Journal of Infectious Diseases 191: 2138–2148.
Kessler, W., T. Traeger, A. Westerholt, F. Neher, M. Mikulcak, A. Muller, S. Maier, and C.D. Heidecke. 2006. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbeck’s Archives of Surgery 391: 83–87.
van Westerloo, D.J., I.A. Giebelen, S. Florquin, M.J. Bruno, G.J. Larosa, L. Ulloa, K.J. Tracey, and T. van der Poll. 2006. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130: 1822–1830.
Guarini, S., D. Altavilla, M.M. Cainazzo, D. Giuliani, A. Bigiani, H. Marini, G. Squadrito, L. Minutoli, A. Bertolini, R. Marini, et al. 2003. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194.
Bernik, T.R., S.G. Friedman, M. Ochani, R. DiRaimo, S. Susarla, C.J. Czura, and K.J. Tracey. 2002. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. Journal of Vascular Surgery 36: 1231–1236.
Huston, J.M., M. Gallowitsch-Puerta, M. Ochani, K. Ochani, R. Yuan, M. Rosas-Ballina, M. Ashok, R.S. Goldstein, S. Chavan, V.A. Pavlov, et al. 2007. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Critical Care Medicine 35: 2762–2768.
van Westerloo, D.J., I.A. Giebelen, J.C. Meijers, J. Daalhuisen, A.F. de Vos, M. Levi, and T. van der Poll. 2006. Vagus nerve stimulation inhibits activation of coagulation and fibrinolysis during endotoxemia in rats. Journal of Thrombosis and Haemostasis 4: 1997–2002.
Wang, H., M. Yu, M. Ochani, C.A. Amella, M. Tanovic, S. Susarla, J.H. Li, H. Wang, H. Yang, L. Ulloa, et al. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388.
Pavlov, V.A., M. Ochani, L.H. Yang, M. Gallowitsch-Puerta, K. Ochani, X. Lin, J. Levi, W.R. Parrish, M. Rosas-Ballina, C.J. Czura, et al. 2007. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Critical Care Medicine 35: 1139–1144.
Zhang, Y., and J. Li. 2012. Carbachol ameliorates lipopolysaccharide-induced intestinal epithelial tight junction damage by down-regulating NFkappabeta and myosin light-chain kinase pathways. Biochemical and Biophysical Research Communications 428: 321–326.
Shimizu, S., T. Akiyama, T. Kawada, Y. Sata, M. Mizuno, A. Kamiya, T. Shishido, M. Inagaki, M. Shirai, S. Sano, and M. Sugimachi. 2012. Medetomidine, an α2-adrenergic agonist, activates cardiac vagal nerve through modulation of baroreflex control. Circulation Journal 76: 152–159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, H., Hu, B., Li, Z. et al. Dexmedetomidine Controls Systemic Cytokine Levels through the Cholinergic Anti-inflammatory Pathway. Inflammation 37, 1763–1770 (2014). https://doi.org/10.1007/s10753-014-9906-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9906-1