Abstract
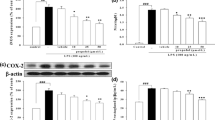
Monocyte chemoattractant protein-1 (MCP-1) is a cytokine that mediates the influx of cells to sites of inflammation. Our group recently reported that propofol exerted an anti-inflammatory effect and could inhibit lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines. However, the effect and possible mechanisms of propofol on MCP-1 expression remain unclear. LPS-stimulated HepG2 cells were treated with 50 μM propofol for 0, 6, 12, and 24 h, respectively. The transcript and protein levels were measured by real-time quantitative PCR and Western blot analyses, respectively. We found that propofol markedly decreased both MCP-1 messenger RNA (mRNA) and protein levels in LPS-stimulated HepG2 cells in a time-dependent manner. Expression of apolipoprotein M (apoM) and forkhead box protein A2 (foxa2) was increased by propofol treatment in HepG2 cells. In addition, the inhibitory effect of propofol on MCP-1 expression was significantly abolished by small interfering RNA against apoM and foxa2 in LPS-stimulated HepG2 cells. Propofol attenuates LPS-induced MCP-1 production through enhancing apoM and foxa2 expression in HepG2 cells.



Similar content being viewed by others
REFERENCES
Marik, P.E. 2004. Propofol: Therapeutic indications and side-effects. Current Pharmaceutical Design 10: 3639–3649.
Vasileiou, I., T. Xanthos, E. Koudouna, D. Perrea, C. Klonaris, et al. 2009. Propofol: A review of its non-anaesthetic effects. European Journal of Pharmacology 605: 1–8.
Acquaviva, R., A. Campisi, P. Murabito, G. Raciti, R. Avola, et al. 2004. Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: An alternative protective mechanism. Anesthesiology 101: 1363–1371.
Sanchez-Conde, P., J.M. Rodriguez-Lopez, J.L. Nicolas, F.S. Lozano, F.J. Garcia-Criado, et al. 2008. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesthesia and Analgesia 106: 371–378.
Mangge, H., K. Becker, D. Fuchs, and J.M. Gostner. 2014. Antioxidants, inflammation and cardiovascular disease. World Journal of Cardiology 6: 462–477.
Golia, E., G. Limongelli, F. Natale, F. Fimiani, V. Maddaloni, et al. 2014. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Current Atherosclerosis Reports 16: 435.
Kolattukudy, P.E., and J. Niu. 2012. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circulation Research 110: 174–189.
Chiu, W.T., Y.L. Lin, C.W. Chou, and R.M. Chen. 2009. Propofol inhibits lipoteichoic acid-induced iNOS gene expression in macrophages possibly through downregulation of toll-like receptor 2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chemico-Biological Interactions 181: 430–439.
Hsing, C.H., M.C. Lin, P.C. Choi, W.C. Huang, J.I. Kai, et al. 2011. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKbeta/NF-kappaB signaling. PloS One 6: e17598.
Chen, R.M., T.G. Chen, T.L. Chen, L.L. Lin, C.C. Chang, et al. 2005. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Annals of the New York Academy of Sciences 1042: 262–271.
Ma, X., Y.W. Hu, Z.L. Zhao, L. Zheng, Y.R. Qiu, et al. 2013. Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1α-dependent manner. Archives of Biochemistry and Biophysics 533: 1–10.
Strecker, J.K., J. Minnerup, B. Gess, E.B. Ringelstein, W.R. Schabitz, et al. 2011. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PloS One 6: e25863.
Panasiuk, A., D. Prokopowicz, and B. Panasiuk. 2004. Monocyte chemotactic protein-1 and soluble adhesion molecules as possible prognostic markers of the efficacy of antiviral treatment in chronic hepatitis C. World Journal of Gastroenterology 10: 3639–3642.
Takada, Y., T. Hisamatsu, N. Kamada, M.T. Kitazume, H. Honda, et al. 2010. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. Journal of Immunology 184: 2671–2676.
Niu, J., and P.E. Kolattukudy. 2009. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clinical Science (London) 117: 95–109.
Deshmane, S.L., S. Kremlev, S. Amini, and B.E. Sawaya. 2009. Monocyte chemoattractant protein-1 (MCP-1): An overview. Journal of Interferon and Cytokine Research 29: 313–326.
O’Hayre, M., C.L. Salanga, T.M. Handel, and S.J. Allen. 2008. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. The Biochemical Journal 409: 635–649.
Shin, W.S., A. Szuba, and S.G. Rockson. 2002. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis 160: 91–102.
Xu, N., and B. Dahlback. 1999. A novel human apolipoprotein (apoM). Journal of Biological Chemistry 274: 31286–31290.
Duan, J., B. Dahlback, and B.O. Villoutreix. 2001. Proposed lipocalin fold for apolipoprotein M based on bioinformatics and site-directed mutagenesis. FEBS Letters 499: 127–132.
Sevvana, M., J. Ahnstrom, C. Egerer-Sieber, H.A. Lange, B. Dahlback, et al. 2009. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. Journal of Molecular Biology 393: 920–936.
Luo, G., X. Zhang, P. Nilsson-Ehle, and N. Xu. 2004. Apolipoprotein M. Lipids in Health and Disease 3: 21.
Richter, S., D.Q. Shih, E.R. Pearson, C. Wolfrum, S.S. Fajans, et al. 2003. Regulation of apolipoprotein M gene expression by MODY3 gene hepatocyte nuclear factor-1alpha: Haploinsufficiency is associated with reduced serum apolipoprotein M levels. Diabetes 52: 2989–2995.
Wolfrum, C., M.N. Poy, and M. Stoffel. 2005. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nature Medicine 11: 418–422.
Tolle, M., A. Pawlak, M. Schuchardt, A. Kawamura, U.J. Tietge, et al. 2008. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arteriosclerosis, Thrombosis, and Vascular Biology 28: 1542–1548.
Feingold, K.R., J.K. Shigenaga, L.G. Chui, A. Moser, W. Khovidhunkit, et al. 2008. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199: 19–26.
Ang, S.L., A. Wierda, D. Wong, K.A. Stevens, S. Cascio, et al. 1993. The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF3/forkhead proteins. Development 119: 1301–1315.
Sund, N.J., M.Z. Vatamaniuk, M. Casey, S.L. Ang, M.A. Magnuson, et al. 2001. Tissue-specific deletion of Foxa2 in pancreatic beta cells results in hyperinsulinemic hypoglycemia. Genes and Development 15: 1706–1715.
Lee, C.S., J.R. Friedman, J.T. Fulmer, and K.H. Kaestner. 2005. The initiation of liver development is dependent on Foxa transcription factors. Nature 435: 944–947.
Mirosevich, J., N. Gao, A. Gupta, S.B. Shappell, R. Jove, et al. 2006. Expression and role of Foxa proteins in prostate cancer. Prostate 66: 1013–1028.
Wolfrum, C., J.J. Howell, E. Ndungo, and M. Stoffel. 2008. Foxa2 activity increases plasma high density lipoprotein levels by regulating apolipoprotein M. Journal of Biological Chemistry 283: 16940–16949.
Zhao, J.Y., Y.W. Hu, S.F. Li, Y.R. Hu, X. Ma, et al. 2014. Dihydrocapsaicin down-regulates apoM expression through inhibiting Foxa2 expression and enhancing LXRα expression in HepG2 cells. Lipids in Health and Disease 13: 50.
Ziraldo, C., Y. Vodovotz, R.A. Namas, K. Almahmoud, V. Tapias, et al. 2013. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PloS One 8: e79804.
Tacke, F. 2012. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis & Tissue Repair 5: S27.
ACKNOWLEDGMENTS
This study was supported by the National Natural Sciences Foundation of China (grant numbers 81271905, 81301489, and 81472009), Science and Technology Planning Project of Guangdong Province (2011B031800090), and Medical Scientific Research Foundation of Guangdong Province (A2011374).
Conflict of Interest
The authors declare no conflicts of interest associated with this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, X., Zhao, JY., Zhao, ZL. et al. Propofol Attenuates Lipopolysaccharide-Induced Monocyte Chemoattractant Protein-1 Production Through Enhancing apoM and foxa2 Expression in HepG2 Cells. Inflammation 38, 1329–1336 (2015). https://doi.org/10.1007/s10753-014-0104-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0104-y