Abstract
The translocation of non-indigenous fishes into lowland rives can result in invasive populations establishing and dispersing. Because non-indigenous fishes can cause ecological harm, it is important to understand their trophic relationships and the effects they may have on native fishes. We assessed the trophic ecology of a translocated chub Squalius cephalus population in the River Frome, a lowland chalk stream in Southern England, using bulk stable isotope (δ13C, δ15N) metrics, and compared the results with those derived from indigenous chub populations. The isotopic niche sizes of all fishes in the River Frome were substantially larger in the tidal versus non-tidal site, with the δ13C values suggesting some fish were foraging further downstream of their sampling point in areas that had greater tidal and salinity influences. Inter-specific comparisons of isotopic niches revealed a consistent pattern of similar niche size and overlap between chub and the trophically analogous dace Leuciscus leuciscus. These isotopic relationships between chub and dace were then also apparent in the indigenous populations of these fishes. These results suggest that the colonisation of this lowland river by translocated chub is being facilitated by their isotopic relationships with other fishes that are similar to those observed in their native range.
Similar content being viewed by others
Introduction
When an alien fish species is introduced or translocated directly into a river catchment then the introduced propagules have the potential to establish a population which can disperse (Dominguez Almela et al., 2020, 2022). These fish populations can then have ecological impacts on native species, which develop through processes including predation and competition (Gozlan et al., 2010). Predicting which introduced fishes will establish, disperse and impact native species remains a major ecological challenge (Copp et al., 2009; Tran et al., 2015), especially given that species-rich riverine fish communities can inhibit these processes through biological resistance (Britton, 2022).
The magnitude and direction of ecological impact that arises from competitive interactions depends on how the non-native and native fishes interact trophically, such as whether they exploit similar prey resources and so converge in resource use or largely exploit different resources and so diverge (Jackson et al., 2012; Tran et al., 2015). If there is convergence in resource use within the species, their trophic niche sizes and positions will be similar, with potential for the species to compete in the prey resources are limiting (Britton, 2022). If these competitive interactions are asymmetric then the consequences for the inferior competitor can include depressed growth rates, reduced body condition and trophic niche displacement (Cucherousset et al., 2012; Kakareko et al., 2013). The niche variation hypothesis posits that where increased competitive interactions develop then the competing species become more specialised in their resource use, resulting in smaller trophic niches that are increasingly diverged (Van Valen 1965; Thomson 2004; Olsson et al., 2009). Alternatively, the fishes can develop more generalist diets, characterised by larger trophic niches, with the competing species exploiting a wider dietary base to maintain their energetic requirements (Svanbäck & Bolnick, 2007). If the available prey resources are not being fully exploited by native species then these provides feeding opportunities for non-native species that minimises their inter-specific competitive interactions (Shea & Chesson, 2002; Tran et al., 2015).
Activities associated with recreational angling, such as fish stocking and live bait release, represent a major introduction pathway for freshwater fish (Cambray, 2003; Winfield & Durie, 2004). This introduction pathway includes the translocation of indigenous fishes into areas within regions where they are non-indigenous (Winfield et al., 2008; Britton et al., 2011). For example, the chub Squalius cephalus (Linnaeus, 1758) was translocated (most likely by anglers) into the River Frome, Southern England, in the late 2000s. Their presence in the river has increased the diversity of non-salmonid species available for angling exploitation, although other rivers in the region already have natural chub populations present (Warren et al., 2022). The natural range of chub is from the northern latitudes of Scandinavia to southern latitudes of the Mediterranean (Kottelat & Freyhof, 2007), but with non-native populations in Italy (Haubrock et al., 2021) and Ireland (Caffrey et al., 2008), although the latter population is now eradicated (Caffrey et al., 2018). In the River Frome, angling reports indicated chub were present in catches from 2008 in the lower tidal river. Their upstream dispersal appears to be relatively slow, but their colonisation of the lower river has been facilitated by their exceptionally fast somatic growth rates (Warren et al., 2022).
Chub is considered indigenous to Britain, with a relatively widespread distribution in lowland rivers (Bolland et al., 2007), although their status in south-west England has been uncertain. This contrasts to the functionally analogous dace Leuciscus leuciscus (Linnaeus, 1758), known to be present in rivers such as the Dorset Stour in 500 BC, suggesting their colonisation of these rivers through connections with rivers of mainland Europe following the end of the last glacial maximum (Wheeler, 1977). Indeed, unlike chub, the dace population of the River Frome is considered as indigenous and has previously been the subject of considerable research attention (e.g. Mann, 1974; Mills & Mann, 1985; Mills et al., 1985). The translocation of chub into this river, a species with an omnivorous diet that, while primarily being invertebrate based, can include facultative piscivory (Caffrey et al., 2008), could result in inter-specific competition with trophically analogous species, including other species of the Cyprinidae family, including dace.
Our initial aim was to assess the trophic ecology of translocated chub in the River Frome in relation to the indigenous cyprinid species present [dace, roach Rutilus rutilus (Linnaeus, 1758), rudd Scardinius erythrophthalmus (Linnaeus, 1758)]. The inter-specific trophic relationships were assessed using a stable isotope analysis (SIA)-based approach in two areas of the river, including the tidal river where values of the carbon stable isotope (13C) are often enriched (Winter et al., 2021). The stable isotope approach enabled comparisons of the isotopic niche sizes and extent of niche overlap between the cyprinid species. The isotopic niche is closely related to the trophic niche, but is also influenced by factors including growth rate and metabolism (Jackson et al., 2011; Hette-Tronquart, 2019). The SI approach was based on the analysis of scale tissue, which provides a longer term perspective on dietary resources when compared to other fish tissues, such as dorsal muscle and fin (e.g. Busst & Britton, 2018). We then identified whether the inter-specific trophic relationships patterns apparent between chub and dace in the River Frome (as the two most functionally similar species present) were similar to those of their native populations. This step enabled us to test whether the interactions of the non-indigenous chub with native fishes were similar to their indigenous populations, as has been observed in other translocated fishes in England, including European barbel Barbus barbus (Linnaeus, 1758) (Gutmann Roberts & Britton, 2020).
Materials and methods
River Frome study site (non-indigenous chub)
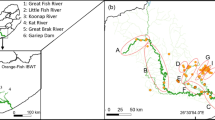
The River Frome is a lowland chalk stream in southern England that flows for approximately 70 km from its source (50.50. 24°N; 02.36. 12°W) to its tidal limit (50.40. 38°N; 02.07. 30°W). It is characterised by pool-riffle habitats throughout much of its length, but transitions to being wider and deeper in its tidal reaches (Simmons et al., 2022). The fish assemblage is dominated numerically by European minnow Phoxinus phoxinus (Linnaeus, 1758), with the river renowned for its population of Atlantic salmon Salmo salar Linnaeus, 1758 and brown trout Salmo trutta Linnaeus, 1758 (Simmons et al., 2020). Indigenous species of the Cyprinidae family, including dace and roach, are found in the lower river. Even in the lower reaches, the river rarely exceeds 20 m width and 2 m depth.
Fish samples were collected in late August and early September 2021. Two areas of the lower river were sampled that were approximately 8 km apart. Site 1, the most upstream site, comprised of the main river channel, a connected millstream and a series of side-streams (Fig. 1). The river was not tidally influenced here. Site 2 was located downstream of the town of Wareham in the tidally influenced section of the river, approximately 4 km upstream of the river’s confluence with Poole Harbour and the sea, meaning fish had the opportunity to forage in tidal areas of the river providing they could tolerate the salinity (Fig. 1). With increasing distance upstream from Site 1, the fish community is dominated by salmonid fishes, with few cyprinid species present in these areas. The physical characteristics of the river at both sites (as depth, flow and width) prevented the use of sampling methods to derive population abundance estimates. Correspondingly, at Site 1, a combination of rod-and-line angling [using bait, usually maggots, Calliphora vomitoria (Linnaeus, 1758)] and electric fishing (Smith Root LR24 back-mounted equipment, pulsed DC, various voltage depending on capture efficiency, water depth and fish recovery rates) was used to sample all habitats, although all of the fish that were used in subsequent analyses were from the millstream and side-streams. At Site 2, fish were sampled by baited rod-and-line angling only due to the inefficiency of electric fishing in that area. Following their capture, all fish were identified to species, measured (fork length, nearest mm) and a sample of scales taken from below the dorsal fin but above the lateral line, where scales were originally taken for the purposes of age and growth analyses for management purposes (Warren et al., 2022). Scales were then stored in paper envelopes that enabled drying, which were then stored in dry, cool conditions (12 to 15 °C). All fish were then returned to the area of river where they were captured. The sample sizes used for stable isotope analysis are provided in Table 1.
Following fish sampling, a common macroinvertebrate fish prey resource was collected from both sites using a sweep net (250 mm wide; 0.3 m bag depth; 250 µm mesh). The most frequently occurring macroinvertebrate at both sites was Gammarus spp., with triplicate samples taken for stable isotope analysis, which were stored frozen before SIA preparation. These samples were used to identify the differences in the stable isotope values of fish putative prey resources between the two sites; if differences between the two sites were not statistically significant then comparisons of the fish stable isotope data could be made between the sites without any correction (Olsson et al., 2009; Britton et al. 2022).
Other sampled rivers (indigenous chub)
Assessment of the isotopic relationships of indigenous chub and dace were through the use of stable isotope data collated from populations in studies completed in the last decade by the authors. Five comparative chub and dace populations were used; these were from the Rivers Avon, Stour and Teme, and two tributaries of the River Great Ouse (‘Great Ouse T1’, ‘Great Ouse T2’; Table 1). These rivers were typically between 8 and 10 m in width, depths to 2 m and were in lowland areas where their fish communities were dominated by cyprinid fishes. The fish samples were all collected in mid to late summer from these rivers. In the Rivers Avon, Teme and Stour, the fish samples were all collected by rod-and-line angling (given the relatively large width and depths of these rivers prevented the effective use of electric fishing), using the same equipment as the River Frome. The two tributaries of the Great Ouse were sampled by generator powered electric fishing (Electracatch; voltage dependent on capture efficiency and habitat sampled), as these were shallower and enabled fishing by wading. At all sites, the captured fish were identified to species, measured (fork length, nearest mm) and a sample of scales taken from below the dorsal fin and above the lateral line that were initially used for age and growth analysis before being used for stable isotope analysis (as already described for the Frome samples above).
Stable isotope analysis
All of the fish scale material and the River Frome Gammarus spp. samples were dried to constant mass (approximately 5 mg) of at 60 °C before analysis at the Cornell University Stable Isotope Laboratory (New York, USA) for δ13C and δ15N in a Thermo Delta V isotope ratio mass spectrometer (Thermo Scientific, USA) interfaced to a NC2500 elemental analyser (CE Elantach Inc., USA). Analytical precision of the δ13C and δ15N sample runs was estimated against an internal standard sample of animal (deer) material every 10 samples, with the overall standard deviation estimated at 0.08 and 0.04 ‰, respectively. Ratios of C:N indicated no requirement for lipid normalisation (generally 3.5 to 3.9) (Winter & Britton, 2021).
The δ13C and δ15N data for each species and site (Tables 1, 2) were initially tested for the influence of fish length using linear regression, followed by testing for differences in both δ13C and δ15N between the species at each site using a generalised linear model (GLM), where species was the response variable, δ13C or δ15N was the fixed factor and fish length was the covariate. Reported test results were the significance of the model and of the differences in mean δ13C and δ15N between the species (from linearly independent pairwise comparisons, with Bonferroni correction for multiple comparisons). The δ13C and δ15N data were then used to calculate the isotopic niche of each fish species at the sites using standard ellipse areas (SEA) in the SIBER package in R (Jackson et al., 2011, 2012), where SEAs are a bivariate measure of the distribution of individuals in isotopic space. As the ellipses enclose the core 40% of data, they represent the typical resource use of the analysed population (Jackson et al., 2011). The Bayesian estimate of SEA (SEAB) was used to test differences in isotopic niche sizes between the species at both sites, calculated using a Markov chain Monte Carlo simulation (104 iterations per category) (Jackson et al., 2011, 2012). Differences in the sizes of isotopic niches (as SEAB) of the species were evaluated in SIBER by calculating the probability that the relative posterior distributions of the niche sizes were significantly smaller or larger between the species (α = 0.05) (Jackson et al., 2011, 2012). Thus, no overlap in the 95% credible intervals of SEAB indicated whether chub had a significantly smaller or larger niche size versus the comparator species. The extent of overlap in the isotopic niches (as SEAB) between species at each site were then determined as 95% credible intervals, also calculated in SIBER. For presenting standard ellipse areas on plots, values of SEAc were calculated in SIBER (subscript ‘c’ indicates a small sample size correction was used; Jackson et al., 2012), as this provided a simpler visual representation of the data distribution and niche positions. However, all evaluations were based on the Bayesian estimates.
All data analyses were completed in R version 4.0.5 (R Development Core Team, 2021). Where error is provided around mean values, it represents 95% confidence limits unless otherwise stated.
Results
Fish length–stable isotope relationships
In the River Frome sites, the lengths of chub and dace in Site 1 and Site 2 were substantially larger than roach (and of rudd in Site 2) (Table 1). The relationships of fork length versus both δ13C and δ15N of all species in both sites were not statistically significant, with the exception of roach δ15N in Site 2, where increased length resulted in more enriched 15N (Table 2). In Site 1, the GLM indicated that δ13C and δ15N did not differ significantly among the species (Table 3). In contrast, the GLM indicated that in site 2, there were significant differences in both δ13C and δ15N of the species (Table 3), where pairwise comparisons revealed that both δ13C and δ15N of chub differed significantly from roach and rudd (P < 0.01 in all cases), but not with dace (P = 0.19, 0.99, respectively).
In the other rivers, the effect of fish length on δ13C was only significant for chub in Great Ouse T1; for δ15N, it was significant for dace in Great Ouse T1 and chub in both the River Stour and Teme (Table 2). The GLMs indicated that differences in δ13C and δ15N between the two species were significant in the Great Ouse T1 and T2, and the River Teme, where length was a significant covariate in Great Ouse T1 (δ13C, δ15N), and the Teme (δ13C only) (Table 3).
Stable isotopic niche sizes and overlap
In the two River Frome sites, the differences in the SI values of the Gammarus spp. did not overlap in their 95% CI (Site 1 vs Site 2: δ13C: − 31.90 ± 0.96 vs − 30.57 ± 0.27; δ15N: vs 7.67 ± 0.19 vs 7.92 ± 0.44 ‰). Correspondingly, the fish isotopic niches could be compared across the two sites without correction. The isotopic niches (as SEAB) of the fishes in Site 1 were all relatively similar in size (Fig. 2), with substantial overlaps in their 95% credible intervals (Table 4). The positions of these niches in isotopic space were also similar between the species, with chub predicted to be sharing 52 to 97% of their niche with dace, and 61 to 99% of their niche with roach (Table 4; Fig. 3). In Site 2, roach had significantly larger isotopic niches than both chub and dace, but not rudd (Fig. 2). The isotopic niche of chub overlapped substantially with dace (up to 98%), but the overlap between chub and the other species was much lower (Table 4; Fig. 3). It was apparent that the species in Site 2 were had greater variability in their SI data than in Site 1, with some fish in Site 2 having enriched values of both 13C and 15N (Figs. 2, 3), resulting in substantially larger isotopic niches (Table 4).
Distribution of the isotopic niche size for each sampled species (chub Squalius cephalus, dace Leuciscus leuciscus, roach Rutilus rutilus and rudd Scardinius erythrophthalmus) in Site 1 and Site 2 of the River Frome, where for each species and site, the horizontal lines represent the credible intervals of the niches (10th, 25th, 75th, 90th), the blue circle represents the mean and the red cross the modal value
Relationships of δ13C and δ15N and core isotopic niches of chub Squalius cephalus (black circle/ solid line), dace Leuciscus leuciscus (black triangle/ small dashed line), roach Rutilus rutilus (black square/medium dashed line) and rudd Scardinius erythrophthalmus (black cross/ largest dashed line) at Site 1 (top) and Site 2 (bottom). Note that the core isotopic niches of each species were calculated by SEAC rather than SEAB (both for presentation purposes only; see Table 4 for 95% credible intervals of the core niches)
As with chub and dace in the River Frome, comparisons of the isotopic niche sizes between the native populations of dace and chub revealed these were not significantly different within each river (Table 1; Figs. 2, 4). Similarly, there was overlap in the isotopic niches of the two species, although this varied between the rivers, with 95% credible intervals ranging from 23 to 74% in the River Teme to 69 to 100% in the Great Ouse T2 (Table 4; Fig. 4).
Relationships of δ13C and δ15N and core isotopic niches of chub Squalius cephalus (black square/ solid line) and dace Leuciscus leuciscus (black triangle/dashed line) from five rivers where both species are native. Note differences in axes values and that the core isotopic niches of each species were calculated by SEAC rather than SEAB (both for presentation purposes only; see Table 4 for 95% credible intervals of the core niches)
Discussion
The trophic relationships of translocated chub in the River Frome revealed that there were considerable overlaps in their isotopic niches with all species in Site 1, but only with dace in Site 2. This niche overlap with dace was consistent with the two species being trophically analogous. The overlapping isotopic niches of chub and dace also had some consistency with sympatric native populations from other rivers in England, where similar relationships were generally evident in the 95% credible intervals around the standard ellipse areas. In general, length was not always a strong predictor of δ13C and δ15N values of either species, but with evidence that in some rivers, enriched values of these isotopes were significantly correlated with fish length (e.g. roach in Site 2 of the River Frome), suggesting some ontogenetic dietary shifts and that overlaps in isotopic niches might have only been apparent for specific length ranges of the species.
There was an unexpected and substantial difference in the size of the isotopic niches of all of the analysed species between Sites 1 and 2 of the River Frome, where the niches at Site 2 were consistently larger and had a much greater range of values of δ13C and δ15N, especially in roach. This was despite the SI values of the Gammarus spp. (as a putative fish prey resource) being similar in the areas where fish were sampled. Thus, some of the fish in Site 2 were feeding on prey resources that were substantially enriched in 13C and which did not include the Gammarus spp. sampled in the fish sampling area.
It was considered likely that these 13C-enriched fish in Site 2 of the River Frome were feeding in areas downstream of the sampling area, i.e. towards Poole Harbour. This salinity of this area of the tidal river varies semi-diurnally (according to the tidal cycle) (Humphreys, 2005). This salinity variation becomes more pronounced as it nears Poole Harbour, with the river close to the confluence with the harbour being partially mixed but with harbour itself being vertically homogenous and considered an estuary (Humphreys, 2005). While this means the fish were unlikely to be able to enter the harbour itself due to excessive salinity levels, they were considered as most likely moving into the lower river to forage, perhaps to exploit relatively rich prey resources that were not being fully exploited by other fishes. Indeed, in tidal rivers generally, SI values of macroinvertebrates (e.g. Gammarus spp.) and cyprinid fishes (e.g. roach) tend to be increasingly enriched in both 13C and 15N, but especially 13C that can help discriminate between foraging areas of fresh water (depleted 13C, e.g. < − 26.0 ‰) and those further downstream where salinity is higher (enriched 13C; e.g. > − 22.0 ‰; Nolan et al., 2019; Winter et al., 2021). With scales providing a longer temporal dietary perspective than muscle and fin tissues (Busst & Britton, 2018), then it can be assumed that the fish feeding in these downstream tidal areas were doing so over extended time periods, i.e. as part of a foraging strategy. Despite their apparent foraging in the tidal reaches, the evidence from Site 2 SI data was that these fish were also mixing with individuals that primarily forage in fresh waters (the individuals with relatively depleted 13C). These results suggest considerable individual specialisation in the foraging of these fishes (Araújo et al., 2011).
The suggestion that some individual cyprinid fishes were foraging in lower tidal river could not be tested further here. The only cyprinid species in the River Frome where tracking studies have been completed is dace, where the fish were sampled, tagged and released at Site 1 in work completed in the 1990s. The results of these studies indicated that the dace had clearly defined daytime and night-time habitats, but with the distances moved between these being only up to 680 m (i.e. these movements did not involve movements between Site 1 and Site 2 across 24 h periods) (Clough & Ladle, 1997). Conversely, a further study indicated that although the downstream distance moved by most dace was rarely > 1 km, a small proportion of individuals did move as far as 9 km downstream (i.e. from Site 1 to Site 2) (Clough & Beaumont, 1998), indicating that at least some dace in the river are capable of making relatively large movements from the non-tidal to tidal areas of the river (and to presumably forage there). Notwithstanding, all of the fish analysed at Site 1 had strong freshwater SI values and, in contrast, the values and range of δ13C of the fishes at Site 2 have already been suggested as indicating some individuals move into the lower tidal river to forage. However, in the absence of data on both the fish putative prey items further downstream of Site 2 (an area which is largely inaccessible) and on fish movements in the lower river, this remains speculative but worthy of further work.
The isotopic niche overlap between the translocated chub and the indigenous fishes of the River Frome raises questions over their potential ecological impacts. Any overlap in isotopic niches between species suggests some sharing of prey resources (or at least prey with similar isotopic signatures) and thus the potential for competitive interactions, especially where these prey resources are limiting, with potential for inferior competitors to be displaced from their niche (Cucherousset et al., 2012; Kakareko et al., 2013). There is, however, no evidence to suggest that the native cyprinid fishes have been displaced by chub in the river, given the niche overlaps and isotopic relationships were relatively consistent with other rivers where the species are naturally sympatric. Moreover, the river is considered to be highly productive in the context of fish growth. For examples, juvenile Atlantic salmon Salmo salar tend to emigrate to sea as smolts are age 1 + , whereas in most rivers of similar latitude, smoltification occurs at least age 2+ years (Simmons et al., 2021). Indeed, the colonisation of the River Frome by chub has already been described by Warren et al. (2022) as being facilitated by their very fast growth. The results here on the isotopic ecology also suggest that their invasiveness might be further facilitated by their stable isotope ecology (and by extension, their feeding ecology) being similar in this translocated population and some of their native populations, i.e. they are expressing similar traits in both their invasive and natural range. The expression of these ‘pre-adapted’ traits is considered to generally facilitate the invasion of introduced species as there is no requirement to adapt to the novel conditions (Catford et al., 2009), and has been observed ißn translocated fishes in England, including European barbel in western England (Gutmann Roberts & Britton, 2020). Notwithstanding these inferences, it is acknowledged that they are based on two sites that were sampled in one summer only. Moreover, greater insights into the trophic ecology of the fishes would have been gained through comprehensive SIA of putative prey items and their application to mixing models to predict diet composition (Nolan et al. 2019). However, the application of stable isotope mixing models can be problematic where the SI data are similar between putative prey resources (as the model cannot easily separate their dietary contributions). Moreover, while collecting putative prey resources in the vicinity of Sites 1 and 2 was possible, the areas downstream to Site 2 (where the fish with enriched 13C values were considered to be foraging) were inherently difficult to sample due to its tidal nature and general inaccessibility from the riparian zone, and so could not be completed here. Rather than using SI mixing models to predict data composition, short-term dietary analyses based on stomach contents analyses could have been used, including DNA barcoding approaches. However, short- versus long-term dietary studies do not always correlate strongly (e.g. Locke et al., 2013) and, when coupled with stomach contents analyses generally being a destructive sampling method, it is not clear how much added value this method would have added to the stable isotope approach that was used.
In summary, the translocation of this chub population into the lower reaches of this productive chalk stream has resulted in the establishment of a population that is dispersing upstream. The results revealed that foraging by the fish populations in the lower river resulted in high dietary specialisations, where individuals differed in the extent of their 13C enrichment and so in the extent of their non-tidal versus tidal foraging. Some overlaps in isotopic niches were evident between chub and the other fishes in the River Frome (as was also detected in naturally sympatric populations), but with the very fast growth of these species in the river (Warren et al., 2022) suggesting that the fish were not competing for limited resources.
Data availability
Data are available from the corresponding author on reasonable request.
References
Araújo, M. S., D. I. Bolnick & C. A. Layman, 2011. The ecological causes of individual specialisation. Ecology Letters 14: 948–958.
Bašić, T. & J. R. Britton, 2016. Characterizing the trophic niches of stocked and resident cyprinid fishes: consistency in partitioning over time, space and body sizes. Ecology and Evolution 6: 5093–5104.
Bolland, J.D., J.R. Britton, & I.G. Cowx. 2007. Lifetime consequences of variable 0 year group length in riverine populations of chub Leuciscus cephalus (L.). Journal of Fish Biology 71(6): 1810–1819.
Britton, J. R., 2022. Contemporary perspectives on the ecological impacts of invasive freshwater fishes. Journal of Fish Biology. https://doi.org/10.1111/jfb.15240.
Britton, J. R., R. E. Gozlan & G. H. Copp, 2011. Managing non-native fish in the environment. Fish and Fisheries 12: 256–274. https://doi.org/10.1111/j.1467-2979.2010.00390.x.
Britton, J. R., J. Cucherousset & V. Dominguez Almela, 2022. Novel trophic subsidies from recreational angling transform the trophic ecology of freshwater fishes. Journal of Applied Ecology 59: 2373–2385.
Busst, G. M. & J. R. Britton, 2018. Tissue-specific turnover rates of the nitrogen stable isotope as functions of time and growth in a cyprinid fish. Hydrobiologia 805: 49–60.
Caffrey, J. M., S. Acevedo, K. Gallagher & J. R. Britton, 2008. Chub (Leuciscus cephalus): a new potentially invasive fish species in Ireland. Aquatic Invasions. 3: 201–209. https://doi.org/10.3391/ai.2008.3.2.11.
Caffrey, J. M., K. Gallagher, D. Broughan & J. T. Dick, 2018. Rapid response achieves eradication – chub in Ireland. Management of Biological Invasions. 9: 475. https://doi.org/10.3391/mbi.2018.9.4.10.
Cambray, J. A., 2003. The global impact of alien trout species – a review; with reference to their impact in South Africa. African Journal of Aquatic Science 28: 61–67.
Catford, J. A., R. Jansson & C. Nilsson, 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions. 15: 22–40. https://doi.org/10.1111/j.1472-4642.2008.00521.x.
Clough, S. & W. R. Beaumont, 1998. Use of miniature radio-transmitters to track the movements of dace, Leuciscus leuciscus (L.) in the River Frome, Dorset. In Advances in Invertebrates and Fish Telemetry. Springer, Dordrecht, 89–97. https://doi.org/10.1007/978-94-011-5090-3_11.
Clough, S. & M. Ladle, 1997. Diel migration and site fidelity in a stream-dwelling cyprinid, Leuciscus leuciscus. Journal of Fish Biology 50: 1117–1119. https://doi.org/10.1111/j.1095-8649.1997.tb01635.x.
Copp, G. H., L. Vilizzi, J. Mumford, G. V. Fenwick, M. J. Godard & R. E. Gozlan, 2009. Calibration of FISK, an invasiveness screening tool for nonnative freshwater fishes. Risk Analysis: An International Journal 29: 457–467. https://doi.org/10.1111/j.1539-6924.2008.01159.x.
Cucherousset, J., S. Bouletreau, A. Martino, J. M. Roussel & F. Santoul, 2012. Using stable isotope analyses to determine the ecological effects of non-native fishes. Fisheries Management and Ecology. 19: 111–119. https://doi.org/10.1111/j.1365-2400.2011.00824.x.
Dominguez Almela, V., S. C. Palmer, D. Andreou, P. K. Gillingham, J. M. Travis & J. R. Britton, 2020. Integrating an individual-based model with approximate Bayesian computation to predict the invasion of a freshwater fish provides insights into dispersal and range expansion dynamics. Biological Invasions 22: 1461–1480. https://doi.org/10.1007/s10530-020-02197-6.
Dominguez Almela, V., S. C. Palmer, D. Andreou, P. K. Gillingham, J. M. Travis & J. R. Britton, 2022. Predicting the influence of river network configuration, biological traits and habitat quality interactions on riverine fish invasions. Diversity and Distributions. 28: 257–270. https://doi.org/10.1111/ddi.13459.
Gozlan, R. E., J. R. Britton, I. Cowx & G. H. Copp, 2010. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology. 76: 751–786. https://doi.org/10.1111/j.1095-8649.2010.02566.x.
Gutmann Roberts, C. & J. R. Britton, 2018. Trophic interactions in a lowland river fish community invaded by European barbel Barbus barbus (Actinopterygii, Cyprinidae). Hydrobiologia 819: 259–273.
Gutmann Roberts, C. & J. R. Britton, 2020. Spawning strategies in cypriniform fishes in a lowland river invaded by non-indigenous European barbel Barbus barbus. Hydrobiologia. 847: 4031–4047. https://doi.org/10.1007/s10750-020-04394-9.
Haubrock, P. J., F. Pilotto, G. Innocenti, S. Cianfanelli & P. Haase, 2021. Two centuries for an almost complete community turnover from native to non-native species in a riverine ecosystem. Global Change Biology. 27: 606–623. https://doi.org/10.1111/gcb.15442.
Hette-Tronquart, N., 2019. Isotopic niche is not equal to trophic niche. Ecology Letters 22: 1987–1989.
Humphreys, J., 2005. 3. Salinity and tides in Poole Harbour: estuary or lagoon? Proceedings in Marine Science 7: 35–47.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology. 80: 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x.
Jackson, M. C., I. Donohue, A. L. Jackson, J. R. Britton, D. M. Harper & J. Grey, 2012. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 7: e31757.
Kakareko, T., J. Kobak, J. Grabowska, L. Jermacz, M. Przybylski, M. Poznańska, D. Pietraszewski & G. H. Copp, 2013. Competitive interactions for food resources between invasive racer goby Babka gymnotrachelus and native European bullhead Cottus gobio. Biological Invasions 15: 2519–2530. https://doi.org/10.1007/s10530-013-0470-7.
Kottelat, M. & J. Freyhof, 2007. Handbook of European Freshwater Fishes, EDP Publications, Cornol:
Locke, S. A., G. Bulté, M. R. Forbes & D. J. Marcogliese, 2013. Estimating diet in individual pumpkinseed sunfish Lepomis gibbosus using stomach contents, stable isotopes and parasites. Journal of Fish Biology 82: 522–537.
Mann, R. H. K., 1974. Observations on the age, growth, reproduction and food of the dace, Leuciscus leuciscus (L.), in two rivers in southern England. Journal of Fish Biology. 6: 237–253. https://doi.org/10.1111/j.1095-8649.1974.tb04542.x.
Mills, C. A. & R. H. K. Mann, 1985. Environmentally-induced fluctuations in year-class strength and their implications for management. Journal of Fisheries Biology. 27: 209–226. https://doi.org/10.1111/j.1095-8649.1985.tb03243.
Mills, C. A., W. R. Beaumont & R. T. Clarke, 1985. Sources of variation in the feeding of larval dace Leuciscus leuciscus in an English river. Transactions of the American Fisheries Society. 114: 519–524.
Nolan, E. T., C. Gutmann Roberts & J. R. Britton, 2019. Predicting the contributions of novel marine prey resources from angling and anadromy to the diet of a freshwater apex predator. Freshwater Biology 64: 1542–1554. https://doi.org/10.1111/fwb.13326.
Olsson, K., P. Stenroth, P. E. R. Nyström & W. Graneli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology. 54: 1731–1740. https://doi.org/10.1111/j.1365-2427.2009.02221.x.
Parker, B., D. Andreou, K. Pabortsava, M. Barrow, I. D. Green & J. R. Britton, 2022. Microplastic loads within riverine fishes and macroinvertebrates are not predictable from ecological or morphological characteristics. Science of the Total Environment 839: 156321.
R Development Core Team, 2021. R: A Language and Environment for Statistical Computing. R Development Core Team, Vienna. http://www.r-project.org/.
Shea, K. & P. Chesson, 2002. Community ecology theory as a framework for biological invasions. Trends in Ecology & Evolution. 17: 170–176. https://doi.org/10.1016/S0169-5347(02)02495-3.
Simmons, O. M., J. R. Britton, P. K. Gillingham & S. D. Gregory, 2020. Influence of environmental and biological factors on the overwinter growth rate of Atlantic salmon Salmo salar Parr in a UK chalk stream. Ecology of Freshwater Fish. 29: 665–678. https://doi.org/10.1111/eff.12542.
Simmons, O. M., S. D. Gregory, P. K. Gillingham, W. D. Riley, L. J. Scott & J. R. Britton, 2021. Biological and environmental influences on the migration phenology of Atlantic salmon Salmo salar smolts in a chalk stream in southern England. Freshwater Biology. 66: 1581–1594. https://doi.org/10.1111/fwb.13776.
Simmons, O. M., J. R. Britton, P. K. Gillingham, M. Nevoux, W. D. Riley, E. Rivot & S. D. Gregory, 2022. Predicting how environmental conditions and smolt body length when entering the marine environment impact individual Atlantic salmon Salmo salar adult return rates. Journal of Fish Biology 101: 378–388.
Svanbäck, R. & D. I. Bolnick, 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society B: Biological Sciences 274: 839–844. https://doi.org/10.1098/rspb.2006.0198.
Thomson, D., 2004. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology 85: 458–470.
Tran, T. N. Q., M. C. Jackson, D. Sheath, H. Verreycken & J. R. Britton, 2015. Patterns of trophic niche divergence between invasive and native fishes in wild communities are predictable from mesocosm studies. Journal of Animal Ecology 84: 1071–1080. https://doi.org/10.1111/1365-2656.12360.
Van Valen, L., 1965. Morphological variation and width of ecological niche. The American Naturalist. 99: 377–390. https://doi.org/10.1086/282379.
Warren, B. I., A. Pinder & J. R. Britton, 2022. Age and growth rates of a translocated chub Squalius cephalus chalk-stream population with comparison to indigenous riverine populations in England. Knowledge and Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2022013.
Wheeler, A., 1977. The origin and distribution of the freshwater fishes of the British Isles. Journal of Biogeography 1–24.
Winfield, I. J. & N. C. Durie, 2004. Fish introductions and their management in the English Lake District. Fisheries Management and Ecology 11: 195–201.
Winfield, I. J., J. M. Fletcher & J. B. James, 2008. The Arctic charr (Salvelinus alpinus) populations of Windermere, UK: population trends associated with eutrophication, climate change and increased abundance of roach (Rutilus rutilus). Environmental Biology of Fishes. 83: 25–35. https://doi.org/10.1007/s10641-007-9235-4.
Winter, E. R. & J. R. Britton, 2021. Individual variability in stable isotope turnover rates of epidermal mucus according to body size in an omnivorous fish. Hydrobiologia 848: 363–370. https://doi.org/10.1007/s10750-020-04444-2.
Winter, E. R., A. M. Hindes, S. Lane & J. R. Britton, 2021. Dual-isotope isoscapes for predicting the scale of fish movements in lowland rivers. Ecosphere 12: e03456.
Funding
BICW was supported by an internship funded by the Fishmongers’ Company Fisheries Fund and a studentship funded by the West Country Rivers Trust and Bournemouth University. BP was supported by a studentship awarded by the Fisheries Society of the British Isles. AST was supported by the TÜBİTAK BİDEB (2219 Program).
Author information
Authors and Affiliations
Contributions
JRB and ACP conceived the study, BICW, JRB and ACP collected field samples, BICW, AST and JRB analysed data, BICW and JRB wrote the manuscript, and all authors contributed to editing the manuscript. All authors approve manuscript submission.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The ethical approval process and all regulated procedures were completed under UK Home Office licence P47216841.
Additional information
Handling editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Warren, B.I.C., Pinder, A.C., Parker, B. et al. Trophic relationships of translocated and indigenous chub Squalius cephalus populations with trophically analogous fishes. Hydrobiologia 851, 1291–1303 (2024). https://doi.org/10.1007/s10750-023-05389-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05389-y