Abstract
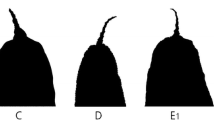
Three well-defined groups, consisting of 15 species, have recently been ascribed to organisms historically identified as the Brachionus plicatilis species complex. One of these groups, the large clade, is composed of two named species (Brachionus plicatilis s.s. and Brachionus manjavacas) and two species identifiers (B. ‘Nevada’ and B. ‘Austria’). B. ‘Austria’ has been confirmed to be B. asplanchnoidis. As no type specimen exists for this species, and the original taxonomic description is lacking in detail, we give a detailed account of this species using material from Obere Halbjochlacke in Austria where B. ‘Austria’ was first identified genetically. Our analysis of B. asplanchnoidis populations was of global scope, an approach that revealed a great degree of morphological variability. However, combining aspects of both the dorsal and ventral surfaces clearly discriminated B. asplanchnoidis from the rest of the large-type members. This approach may prove useful in taxonomic studies of other cryptic species with relatively few morphological features. We also observed a geographic pattern of genetic divergence within B. asplanchnoidis. Average uncorrected COI divergences for a 554-bp fragment of the COI gene ranged from 3.9% within species to 17.5% between species of the large clade and indicate deep divisions within the cryptic species complex.






Similar content being viewed by others
Change history
02 October 2019
The authors of the original publication recognized that, for three of the clones (MAN-L5, LFL2, KOR), the data of two of the raw morphometric measurements contained in Supplementary Material 2 of the article were flipped (the distance between the anterior tips of the 3rd dorsal spines ���b��� and the width of the lorica ���c���). The corrected Supplementary material 2 is provided here. As a consequence, the principal components analysis (PCA) and discriminant analysis (DA) were repeated, and the corrected version of Fig.��3, Tables��4, 5, 6, and 7 are also provided here.
02 October 2019
The authors of the original publication recognized that, for three of the clones (MAN-L5, LFL2, KOR), the data of two of the raw morphometric measurements contained in Supplementary Material 2 of the article were flipped (the distance between the anterior tips of the 3rd dorsal spines ���b��� and the width of the lorica ���c���). The corrected Supplementary material 2 is provided here. As a consequence, the principal components analysis (PCA) and discriminant analysis (DA) were repeated, and the corrected version of Fig.��3, Tables��4, 5, 6, and 7 are also provided here.
References
Abramoff, M. D., P. J. Magalhaes & S. J. Ram, 2004. Image processing with ImageJ. Biophotonics International 11: 36–42.
Ahlstrom, E. H., 1940. A revision of the rotatorian genera Brachionus and Platyias, with descriptions of one new species and two new varieties. Bulletin of the American Museum of Natural History 77: 143–184.
Brown, J. M., S. M. Hedtke, A. R. Lemmon & E. M. Lemmon, 2010. When trees grow too long: investigating the causes of highly inaccurate bayesian branch-length estimates. Systematic Biology 59: 145–161.
Campillo, S., E. M. García-Roger, D. Martínez-Torres & M. Serra, 2005. Morphological stasis of two species belonging to the L-morphotype in the Brachionus plicatilis species complex. Hydrobiologia 546: 181–187.
Charin, N. N., 1947. O novom vide kolovratki is roda Brachionus. Doklady Akademii Nauk SSSR 56: 107–108.
Ciros-Pérez, J., A. Gómez & M. Serra, 2001. On the taxonomy of three sympatric sibling species of the Brachionus plicatilis (Rotifera) complex from Spain, with the description of B. ibericus n.sp. Journal of Plankton Research 23: 1311–1328.
Darriba, D., G. L. Taboada, R. Doallo & D. Posada, 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772.
Drummond, A. J., M. A. Suchard, D. Xie & A. Rambaut, 2012. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973.
Edgar, R. C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797.
Ezard, T., T. Fujisawa & T. G. Barraclough, 2014. splits: SPecies’ LImits by Threshold Statistics. R package version 1.0-19/r51. [available on internet at http://R-Forge.R-project.org/projects/splits/].
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Fontaneto, D., 2014. Molecular phylogenies as a tool to understand diversity in rotifers. International Review of Hydrobiology 99: 178–187.
Fontaneto, D., I. Giordani, G. Melone & M. Serra, 2007. Disentangling the morphological stasis in two rotifer species of the Brachionus plicatilis species complex. Hydrobiologia 583: 297–307.
Fontaneto, D., M. Kaya, E. A. Herniou & T. G. Barraclough, 2009. Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy. Molecular Phylogenetics and Evolution 53: 182–189.
Fu, Y., K. Hirayama & Y. Natsukari, 1991. Morphological differences between two types of the rotifer Brachionus plicatilis O.F. Müller. Journal of Experimental Marine Biology and Ecology 151: 29–41.
Gómez, A. & G. R. Carvalho, 2000. Sex, parthenogenesis and genetic structure of rotifers: microsatellite analysis of contemporary and resting egg bank populations. Molecular Ecology 9: 203–214.
Gómez, A., M. Temprano & M. Serra, 1995. Ecological genetics of a cyclical parthenogen in temporary habitats. Journal of Evolutionary Biology 8: 601–622.
Gómez, A., M. Serra, G. R. Carvalho & D. H. Lunt, 2002. Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera). Evolution 56: 1431–1444.
Horváth, Z. C. F., A. Vad, K. Tóth, E. Zsuga, L. Vörös Boros & R. Ptacnik, 2014. Opposing patterns of zooplankton diversity and functioning along a natural stress gradient: when the going gets tough, the tough get going. Oikos 123: 461–471.
International Commission on Zoological Nomenclature, 1999. International Code of Zoological Nomenclature, 4th edition, 306 pp.
Itigilova, M. Ts., A. Dulmaa & E Yu Afonina, 2014. Zooplankton of lakes of the Uldza and Kerulen River Valleys of northeastern Mongolia. Inland Water Biology 7: 249–258.
Jersabek, C. D. & E. Bolortsetseg, 2010. Mongolian rotifers (Rotifera, Monogononta) – a checklist with annotations on global distribution and autecology. Proceeding of the Academy of Natural Sciences of Philadephia 159: 119–168.
Jersabek, C. D., E. Bolortsetseg & H. L. Taylor, 2010. Mongolian rotifers on microscope slides: instructions to permanent specimen mounts from expedition material. Mongolian Journal of Biological Sciences 8: 51–57.
Koste, W. & R. J. Shiel, 1980. New Rotifera from Australia. Transactions of the Royal Society of South Australia 104: 133–144.
Kutikova, L.A., 1970. Rotifer Fauna USSR. Fauna USSR. 104. Leningrad: Acad. Nauk. SSSR.
Librado, P. & J. Rozas, 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452.
Marshall, D. C., 2010. Cryptic failure of partitioned Bayesian phylogenetic analyses: lost in the land of long trees. Systematic Biology 59: 108–117.
Mills, S., J. Arturo Alcántara-Rodríguez, J. Ciros-Pérez, A. Gómez, A. Hagiwara, K. Hinson Galindo, C. D. Jersabek, R. Malekzadeh-Viayeh, F. Leasi, J.-S. Lee, D. B. M. Welch, S. Papakostas, S. Riss, H. Segers, M. Serra, R. Shiel, R. Smolak, T. W. Snell, C.-P. Stelzer, C. Q. Tang, R. L. Wallace, D. Fontaneto & E. J. Walsh, 2016. Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia (this volume)
Okogwu, O. I., 2010. Seasonal variations of species composition and abundance of zooplankton in Ehoma Lake, a floodplain lake in Nigeria. Revista de Biologia Tropical 58: 171–182.
Oogami, H., 1976. On the morphology of Brachionus plicatilis (in Japanese). Newsletter Izu Branch, Shizuoka Prefectural Fisheries Research Center 18: 2–5.
Ovander, E., N. Iakovenko, V. Trokhymets, Yu Gromova, O. Pashkova & L. Guleikova, 2011. Annotated checklist of monogonont rotifers belonging to the order Ploima (Rotifera: Eurotatoria, Monogonontam Ploima) of Ukraine. Part II. PИБOГOCПOДAPCЬКA HAУКA УКPAÏHИ 3: 46–54.
Papakostas, S., A. Triantafyllidis, I. Kappas & T. J. Abatzopoulos, 2005. The utility of the 16S gene in investigating cryptic speciation within the Brachionus plicatilis species complex. Marine Biology 147: 1129–1139.
Papakostas, S., A. Triantafyllidis, I. Kappas & T. J. Abatzopoulos, 2009. Clonal composition of Brachionus plicatilis s.s. and B. sp. ‘Austria’ hatchery strains based on microsatellite data. Aquaculture 296: 15–20.
Papakostas, S., E. Michaloudi, A. Triantafyllidis, I. Kappas & T. J. Abatzopoulos, 2013. Allochronic divergence and clonal succession: two microevolutionary processes sculpturing population structure of Brachionus rotifers. Hydrobiologia 700: 33–45.
Paradis, E., J. Claude & K. Strimmer, 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290.
Pons, J., T. G. Barraclough, J. Gomez-Zurita, A. Cardoso, D. P. Duran, S. Hazell, S. Kamoun, W. D. Sumlin & A. P. Vogler, 2006. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology 55: 595–609.
Proios, K., E. Michaloudi, S. Papakostas, I. Kappas, K. Vasileiadou & T. J. Abatzopoulos, 2014. Updating the description and taxonomic status of Brachionus sessilis Varga, 1951 (Rotifera: Brachionidae) based on detailed morphological analysis and molecular data. Zootaxa. 3873: 345–370.
Rambaut, A., M. A. Suchard, D. Xie & A. J. Drummond, 2014. Tracer v1.6 [available on internet at http://beast.bio.ed.ac.uk/Tracer].
Riss, S., W. Arthofer, F. M. Steiner, B. C. Schlick-Steiner, M. Pichler, P. Stadler & C.-P. Stelzer, 2016. Do genome size differences within Brachionus asplanchnoidis (Rotifera, Monogononta) cause reproductive barriers among geographic populations? Hydrobiologia (this volume)
Rong, S., H. Segers & H. J. Dumont, 1998. Distribution of Brachionidae (Rotifera, Monogononta) in Inner Mongolian waters. International Review of Hydrobiology 83: 305–310.
Ronquist, F., M. Teslenko, P. van der Mark, D. L. Ayres, A. Darling, S. Höhna, B. Larget, L. Liu, M. A. Suchard & J. P. Huelsenbeck, 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542.
Schlick-Steiner, B. C., B. Seifert, C. Stauffer, E. Christian, R. H. Crozier & F. M. Steiner, 2007. Without morphology, cryptic species stay in taxonomic crypsis following discovery. Trends in Ecology and Evolution 22: 391–392.
Segers, H., 1995. Nomenclature consequences of some recent studies on Brachionus plicatilis (Rotifera, Brachionidae). Hydrobiologia 313(314): 121–122.
Segers, H., 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564: 1–104.
Segers, H., G. Murugan & H. J. Dumont, 1993. On the taxonomy of the Brachionidae: description of Plationus n. gen. (Rotifera, Monogononta). Hydrobiologia 268: 1–8.
Segers, H., W. De Smet, C. Fischer, D. Fontaneto, E. Michaloudi, R. L. Wallace & C. D. Jersabek, 2012. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 3179: 61–68.
Stamatakis, A., P. Hoover & J. Rougemont, 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771.
Stelzer, C. P., S. Riss & P. Stadler, 2011. Genome size evolution at the speciation level: the cryptic species complex Brachionus plicatilis (Rotifera). BMC Evolutionary Biology 11: 90.
Suatoni, E., S. Vicario, S. Rice, T. Snell & A. Caccone, 2006. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer – Brachionus plicatilis. Molecular Phylogenetics and Evolution 41: 86–98.
Sukumaran, J. & M. T. Holder, 2010. DendroPy: a Python library for phylogenetic computing. Bioinformatics 26: 1569–1571.
Tamura, K., G. Stecher, D. Peterson, A. Filipski & S. Kumar, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729.
Tang, C. Q., F. Leasi, U. Obertegger, A. Kieneke, T. G. Barraclough & D. Fontaneto, 2012. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proceedings of the National Academy of Sciences 109: 16208–16212.
Tóth, A., Z. Horváth, C. F. Vad, K. Zsuga, S. A. Nagy & E. Boros, 2014. Zooplankton of the European soda pans: Fauna and conservation of a unique habitat type. International Review of Hydrobiology 99: 255–276.
Xiang, X.-L., Y.-L. Xi, X.-L. Wen, G. Zhang, J.-X. Wang & K. Hu, 2011. Patterns and processes in the genetic differentiation of the Brachionus calyciflorus complex, a passively dispersing freshwater zooplankton. Molecular Phylogenetics and Evolution 59: 386–398.
Yermolaeva, N. I. & O. S. Burmistrova, 2005. Influence of mineralization on zooplankton of the Lake Chany. Cubupckuŭ Эkoлozuлeckuŭ жypнaл 2: 235–247.
Zagorodnyaya, Y. A., E. A. Batogova & N. V. Shadrin, 2008. Long-term transformation of zooplankton in the hypersaline lake Bakalskoe (Crimea) under salinity fluctuations. MOPCЬКИЙ EКOЛOГIЧHИЙ ЖУPHAЛ 7: 41–50.
Acknowledgments
We would like to thank M. Serra and T.W. Snell for providing individuals and resting eggs from clones they keep in their laboratories; M. Pichler for providing technical assistance; A. Herzig for assisting in the collection of samples from Obere Halbjochlacke and Oberer Stinkersee (Austria), and C. Jersabek for the collection of sediments from which MNCHU clones were extracted and for assistance with the identification of OHJ72. This work was partially supported by an EU research project (ROTIGEN, Q5RS-2002-01302), while SP was supported by the Academy of Finland (Grant Number 258048). We gratefully acknowledge the efforts of two anonymous reviewers and D. Fontaneto whose valuable suggestions were extremely helpful to finally shape the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Evangelia Michaloudi, Scott Mills and Spiros Papakostas have contributed equally to this work.
Guest editors: M. Devetter, D. Fontaneto, C. D. Jersabek, D. B. Mark Welch, L. May & E. J. Walsh / Evolving rotifers, evolving science
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michaloudi, E., Mills, S., Papakostas, S. et al. Morphological and taxonomic demarcation of Brachionus asplanchnoidis Charin within the Brachionus plicatilis cryptic species complex (Rotifera, Monogononta). Hydrobiologia 796, 19–37 (2017). https://doi.org/10.1007/s10750-016-2924-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2924-2