Abstract
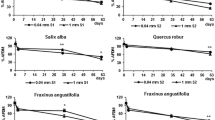
Although habitat size is known to influence both structural and functional properties of ecosystems, there have been few attempts to assess the influence of habitat size on ecosystem processes. Here we investigated the relationships between leaf litter decomposition and ecosystem surface area, macroinvertebrates and physico-chemical factors in five freshwater springs located in Huntingdon County (Pennsylvania, U.S.A.). Leaves of Ulmus americana L. were used to study leaf litter breakdown with the litter-bag technique. Field work was carried out at one sampling station per spring, each with eight replicates per sampling time (3, 20, 40 days), from April to May 2004. American elm leaves decomposed at different rates in the different springs, varying inversely with the spring area. The leaf bags were colonized by 16 taxa of benthic macrofauna, amongst which scrapers and shredders were the most common guild. Macroinvertebrate species richness co-varied with spring area, but not with other physico-chemical variables. Moreover, a significant inverse relationship was observed between American elm leaf decay rate and taxonomic richness. In the studied springs, habitat area was an ecosystem feature indirectly affecting detritus processing by influencing the structure of the detrital food web within the systems.



Similar content being viewed by others
References
Abelho, M., C. Cressa & M. Graça, 2005. Microbial biomass, respiration, and decomposition of Hura crepitans L. (Euphorbiaceae) leaves in a tropical stream. Biotropica 37(3): 397–402.
Anderson, M. J. & C. J. F. ter Braak, 2003. Permutation tests for multifactorial analysis of variance. Journal of Statistical Computation and Simulation 73: 85–113.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVAþ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth, UK: 214 pp.
Ando, M., 1970. Litter fall and decomposition in some evergreen coniferous forests. Japanese Journal of Ecology 2: 1–19.
Baldy, V., E. Chauvet, J. Y. Charcosset & M. O. Gessner, 2002. Microbial dynamics associated with leaves decomposing in the mainstem and floodplain pond of a large river. Aquatic Microbial Ecology 28: 25–36.
Barquìn, J. & R. G. Death, 2006. Spatial patterns of macroinvertebrate diversity in New Zealand springbrooks and rhithral streams. Journal of the North American Benthological Society 25: 768–786.
Basset, A. & D. S. Glazier, 1995. Resource limitation and intraspecific patterns of weight x length variation among spring detritivores. Hydrobiologia 316: 127–137.
Canhoto, C. & M. A. S. Graça, 1996. Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333: 79–85.
Chergui, H. & E. Pattee, 1990. The processing of leaves of trees and aquatic macrophytes in the network of the River Rhone. International Revue of Hydrobiology 75: 281–302.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth, UK: 190 pp.
Cummins, K. W. & M. J. Klug, 1979. Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10: 147–172.
Dangles, O. & B. Malmqvist, 2004. Species richness–decomposition relationships depend on species dominance. Ecology Letters 7: 395–402.
Dumnicka, E., J. Galas & P. Koperski, 2007. Benthic invertebrates in karst springs: does substratum or location define communities? International Revue of Hydrobiology 92: 452–464.
Eichem, A. C., W. K. Dobbs, C. M. Tate & C. Edler, 1993. Microbial decomposition of Elm and Oak leaves in a karst aquifer. Applied and Environmental Microbiology 59: 3592–3596.
Findlay, S. G. & T. L. Arsuffi, 1989. Microbial growth and detritus transformations during decomposition of leaf litter in a stream. Freshwater Biology 21: 261–269.
Fisher, S. G. & G. E. Likens, 1973. Energy flow in Brear Brook, New Hampshire an alternative approach to stream metabolism. Ecological Monographs 43: 421–439.
Galas, J., T. Bednarz, E. Dumnicka, A. Starzecka & K. Wojtan, 1996. Litter decomposition in mountain cave water. Archiv fur Hydrobiologie 138: 199–211.
Gazzera, S. B., K. W. Cummins & G. Salmoiraghi, 1993. Elm and maple processing rates: comparisons between and within streams. Annales de Limnologie 29: 189–202.
Gessner, M. O., E. Chauvet & M. Dobson, 1999. A perspective on leaf litter breakdown in streams. Oikos 85: 377–384.
Glazier, D. S., 1991. The fauna of North American temperate cold springs: patterns and hypotheses. Freshwater Biology 26: 527–542.
Glazier, D. S., 1998. Springs as model systems for ecology and evolutionary biology: a case study of Gammarus minus Say (Amphipoda) in Mid-Appalachian springs differing in pH and ionic content. In Botosaneanu, L. (ed.), Studies in crenobiology: the biology of springs and springbrooks. Backhuys, Leiden: 49–62.
Glazier, D. S., 1999. Variation in offspring investment within and among populations of Gammarus minus Say (Crustacea: Amphipoda) in ten mid-Appalachian springs (U.S.A.). Archiv fur Hydrobiologie 146: 257–283.
Glazier, D. S., 2009. Springs. In Likens, G. E. (ed.), Encyclopedia of inland waters. Elsevier, Oxford: 734–755.
Glazier, D. S. & J. L. Gooch, 1987. Macroinvertebrate assemblages in Pennsylvania (U.S.A.) springs. Hydrobiologia 150: 33–43.
Glazier, D. S., M. T. Horne & M. E. Lehman, 1992. Abundance, body composition and reproductive output of Gammarus minus (Crustacea: Amphipoda) in ten cold springs differing in pH and ionic content. Freshwater Biology 28: 149–163.
Graça, M. A. S., R. C. F. Ferreira & C. N. Coimbra, 2001. Litter processing along a stream gradient: the role of invertebrates and decomposers. Journal of the North American Benthological Society 20: 408–420.
Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), 2005. Methods to Study Litter Decomposition. A Practical Guide. Springer, Netherlands: 329 pp.
Hättenschwiler, S., A. V. Tiunov & S. Scheu, 2005. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology and Systematics 36: 191–218.
Hieber, M. & M. O. Gessner, 2002. Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83: 1026–1038.
Hooker, K. L. & G. R. Marzolf, 1987. Differential decomposition of leaves in grassland and gallery forest reaches of Kings Creek. Transactions of the Kansas Academy of Science 90: 17–24.
Horton, R. T. & A. V. Brown, 1991. Processing of green American Elm leaves in first, third and fifth order reaches of an Ozark stream. Journal of Freshwater Ecology 6: 115–119.
Iversen, T. M., 1988. Secondary production and trophic relationships in a spring invertebrate community. Limnology and Oceanography 33: 582–592.
Jacobson, R. L. & D. Langmuir, 1970. The chemical history of some spring waters in carbonate rocks. Groundwater 8: 5–9.
Jonsson, M. & B. Malmqvist, 2000. Ecosystem process rate increases with animal species richness: evidence from leaf-eating, aquatic insects. Oikos 89: 519–523.
Kodric-Brown, A. & J. H. Brown, 1993. Highly structured fish communities in Australian desert springs. Ecology 74: 1847–1855.
Longhi, D., M. Bartoli & P. Viaroli, 2008. Decomposition of four macrophytes in wetland sediments: organic matter and nutrient decay and associated benthic processes. Aquatic Botany 89: 303–310.
Mancinelli, G., M. L. Costantini & L. Rossi, 2002. Cascading effects of predatory fish exclusion on the detritus-based food web of a lake littoral zone (Lake Vico, central Italy). Oecologia 133: 402–411.
Mancinelli, G., M. L. Costantini & L. Rossi, 2007. Top-down control of reed detritus processing in a lake littoral zone: experimental evidence of a seasonal compensation between fish and invertebrate predation. International Revue of Hydrobiology 92: 117–134.
Matthews, W. J., J. J. Hoover & W. B. Milstead, 1985. Fishes of Oklahoma springs. Southwestern Naturalist 30: 23–32.
McArthur, J. V., J. M. Aho, R. B. Rader & G. L. Mills, 1994. Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. Journal of the North American Benthological Society 13: 57–67.
Menéndez, M., D. Carlucci, M. Pinna, F. A. Comin & A. Basset, 2003. Effect of nutrients on decomposition of Ruppia cirrhosa in a shallow coastal lagoon. Hydrobiologia 506–509: 729–735.
Minshall, G. W., 1967. Role of allochthonous detritus in the trophic structure of a woodland springbrook community. Ecology 48: 139–149.
Odum, H. T., 1957. Trophic structure and productivity of Silver Springs, Florida. Ecological Monographs 27: 55–112.
Petersen, R. C. & K. W. Cummins, 1974. Leaf processing in a woodland stream. Freshwater Biology 4: 343–368.
Post, D. M., M. L. Pace & N. G. Hairston Jr, 2000. Ecosystem size determines food-chain length in lakes. Nature 405: 1047–1049.
Prescott, C. E., L. M. Zabek, C. L. Staley & R. Kabzems, 2000. Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Canadian Journal of Forest Research 30: 1742–1750.
Quintino, V., F. Sangiorgio, F. Ricardo, R. Mamede, A. Pires, R. Freitas, A. M. Rodrigues & A. Basset, 2009. In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuarine Coastal and Shelf Science 85: 497–506.
Ribas, A. C., A. De, M. O. Tanaka & A. L. T. De Souza, 2006. Evaluation of macrofaunal effects on leaf litter breakdown rates in aquatic and terrestrial habitats. Austral Ecology 31: 783–790.
Ruetz, C. R., R. M. Newman & B. Vondracek, 2002. Top-down control in a detritus-based food web: fish, shredders, and leaf breakdown. Oecologia 132: 307–315.
Sangiorgio, F., M. Pinna & A. Basset, 2004. Inter- and intrahabitat variability of plant detritus decomposition in a transitional environment (Lake Alimini, Adriatic Sea). Chemistry and Ecology 20: 353–366.
Shanks, R. E. & J. S. Olson, 1961. First year breakdown of leaf litter in Southern Appalachian forest. Ecology 134: 194–195.
Teal, J. H., 1957. Community metabolism in a temperate cold spring. Ecological Monographs 27: 283–302.
Thompson, P. L. & F. Bärlocher, 1989. Effect of pH on leaf breakdown in streams and in the laboratory. Journal of the North American Benthological Society 8: 203–210.
Vitousek, P. M., L. L. Hoope & H. Andersen (eds), 1995. Islands, Biological Diversity and Ecosystem Function. Springer, Berlin.
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 1997. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277: 102–104.
Wardle, D. A., O. Zackrisson, G. Hörnberg & C. Gallet, 1997. The influence of island area on ecosystem properties. Science 277: 1296–1297.
Webster, J. R. & E. F. Benfield, 1986. Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17: 567–594.
Williams, L. R., M. Taylor & M. L. Warren Jr, 2003. Influence of fish predation on assemblage structure of macroinvertebrates in an intermittent stream. Transactions of the American Fisheries Society 132: 120–130.
Winkelmann, C., S. Worischka, J. H. E. Koop & J. Benndorf, 2007. Predation effects of benthivorous fish on grazing and shredding macroinvertebrates in a detritus-based stream food web. Limnologica 37: 121–128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: P. Viaroli
Rights and permissions
About this article
Cite this article
Sangiorgio, F., Glazier, D.S., Mancinelli, G. et al. How can habitat size influence leaf litter decomposition in five mid-Appalachian springs (USA)? The importance of the structure of the detritivorous guild. Hydrobiologia 654, 227–236 (2010). https://doi.org/10.1007/s10750-010-0390-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0390-9