Abstract
Background
Oxidative stress-induced cell ferroptosis occurs during the pathogenesis of diabetic retinopathy (DR), but the detailed molecular mechanisms are still unclear. The present study aimed to investigate this issue.
Materials and methods
The retinal pigment epithelium (RPE) was treated with high glucose (30 mM) in vitro to mimic the realistic conditions of DR progression in vivo. Cell viability was determined by MTT assay and trypan blue staining assay. Gene expressions were examined by Real-Time qPCR and Western Blot analysis. FCM was used to detect cell apoptosis and ROS generation. Dual-luciferase reporter gene system assay was used to verify the targeting sites.
Results
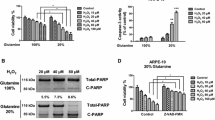
High glucose increased reactive oxygen species (ROS) levels, promoted cell ferroptosis, and suppressed cell proliferation and viability in RPE, which were reversed by co-treating cells with both a ferroptosis inhibitor ferrostatin-1 and an ROS scavenger, N-acetyl-L-Cysteine (NAC). In addition, we screened out a miR-338-3p/ASCT2 (SLC1A5) axis that played an important role in this process. Mechanistically, miR-338-3p targeted the 3’ untranslated regions (3’UTR) of SLC1A5 for its inhibition and degradation, and high glucose downregulated SLC1A5 by upregulating miR-338-3p in RPE cells. Next, the miR-338-3p inhibitor and SLC1A5 overexpression vectors were delivered into the RPE cells, and the following gain- and loss-of-function experiments validated that both miR-338-3p ablation and SLC1A5 upregulation abrogated the regulating effects of high glucose on cell proliferation, viability, ferroptosis and ROS production in RPE cells.
Conclusions
Collectively, data in the present study indicated that targeting the miR-338-3p/SLC1A5 axis could block high glucose-induced ferroptosis in RPE cells.




Similar content being viewed by others
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)).
References
Akbulut H, Ersoy YE, Coskunpinar E, Gucin Z, Yildiz S, Malya FU, Hasturk B, Muslumanoglu M (2019) The role of miRNAs as a predictor of multicentricity in breast cancer. Mol Biol Rep 46(2):1787–1796. https://doi.org/10.1007/s11033-019-04629-6
Altmann C, Schmidt MHH (2018) The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int J Mol Sci 19(1). https://doi.org/10.3390/ijms19010110
Cecilia OM, José Alberto CG, José NP, Ernesto Germán CM, Ana Karen LC, Luis Miguel RP, Ricardo Raúl RR, Daniel A (2019) RC, Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J Diabetes Res, 2019: p. 8562408https://doi.org/10.1155/2019/8562408
Groeneveld Y, Tavenier D, Blom JW, Polak BCP (2019) Incidence of sight-threatening diabetic retinopathy in people with Type 2 diabetes mellitus and numbers needed to screen: a systematic review. Diabet Med 36(10):1199–1208. https://doi.org/10.1111/dme.13908
Gu C, Draga D, Zhou C, Su T, Zou C, Gu Q, Lahm T, Zheng Z, Qiu Q (2019) miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Invest Ophthalmol Vis Sci 60(13):4215–4223. https://doi.org/10.1167/iovs.19-27825
Ikelle L, Naash MI, Al-Ubaidi MR (2019) Oxidative Stress, Diabetic Retinopathy, and Superoxide Dismutase 3. Adv Exp Med Biol 1185:335–339. https://doi.org/10.1007/978-3-030-27378-1_55
Ji H, Yi Q, Chen L, Wong L, Liu Y, Xu G, Zhao J, Huang T, Li B, Yang Y, Li W, Han L, Duan S (2020) Circulating miR-3197 and miR-2116-5p as novel biomarkers for diabetic retinopathy. Clin Chim Acta 501:147–153. https://doi.org/10.1016/j.cca.2019.10.036
Jiang D, Zhou B, Xiong Y, Cai H (2019) miR-135 regulated breast cancer proliferation and epithelial-mesenchymal transition acts by the Wnt/β-catenin signaling pathway. Int J Mol Med 43(4):1623–1634. https://doi.org/10.3892/ijmm.2019.4081
Jo DH, Yun JH, Cho CS, Kim JH, Kim JH, Cho CH (2019) Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia 67(2):321–331. https://doi.org/10.1002/glia.23542
Kajarabille N, Latunde-Dada GO (2019) Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int J Mol Sci 20(19). https://doi.org/10.3390/ijms20194968
Lee JJ, Ishihara K, Notomi S, Efstathiou NE, Ueta T, Maidana D, Chen X, Iesato Y, Caligiana A, Vavvas DG (2020) Lysosome-associated membrane protein-2 deficiency increases the risk of reactive oxygen species-induced ferroptosis in retinal pigment epithelial cells. Biochem Biophys Res Commun 521(2):414–419. https://doi.org/10.1016/j.bbrc.2019.10.138
Li Z, Dong Y, He C, Pan X, Liu D, Yang J, Sun L, Chen P, Wang Q (2019) RNA-Seq Revealed Novel Non-proliferative Retinopathy Specific Circulating MiRNAs in T2DM Patients. Front Genet 10:531. https://doi.org/10.3389/fgene.2019.00531
Liang Z, Lu C, Feng T, Gao X, Tu Y, Yang W, Wang Y (2022) Circ-ADAM9 Promotes High Glucose-Induced Retinal Pigment Epithelial Cell Injury in DR via Regulating miR-338-3p/CARM1 Axis. J Ophthalmol, 2022: p. 2522249https://doi.org/10.1155/2022/2522249
Liu Q, Zhang X, Cheng R, Ma JX, Yi J, Li J (2019) Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress-mediated Wnt/β-catenin pathway activation. Cell Tissue Res 376(2):165–177. https://doi.org/10.1007/s00441-018-2974-z
Luan X, Wang Y (2018) LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3. J Gynecol Oncol 29(6):e. https://doi.org/10.3802/jgo.2018.29.e95
Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O’Connell D, Zhang P, Li Y, Gao T, Ren W, Yang Y (2018) miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ 25(8):1457–1472. https://doi.org/10.1038/s41418-017-0053-8
Martinez B, Peplow PV (2019) MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural Regen Res 14(11):1858–1869. https://doi.org/10.4103/1673-5374.259602
Mei X, Zhang T, Ouyang H, Lu B, Wang Z, Ji L (2019) Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem Pharmacol 159:82–95. https://doi.org/10.1016/j.bcp.2018.11.011
Osanai-Sasakawa A, Hosomi K, Sumitomo Y, Takizawa T, Tomura-Suruki S, Imaizumi M, Kasai N, Poh TW, Yamano K, Yong WP, Kono K, Nakamura S, Ishii T, Nakai R (2018) An anti-ASCT2 monoclonal antibody suppresses gastric cancer growth by inducing oxidative stress and antibody dependent cellular toxicity in preclinical models. Am J Cancer Res 8(8):1499–1513
Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL (2019) Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev, 2019: p. 4940825.https://doi.org/10.1155/2019/4940825
Satari M, Aghadavod E, Mobini M, Asemi Z (2019) Association between miRNAs expression and signaling pathways of oxidative stress in diabetic retinopathy. J Cell Physiol 234(6):8522–8532. https://doi.org/10.1002/jcp.27801
Shao Y, Dong LJ, Takahashi Y, Chen J, Liu X, Chen Q, Ma JX, Li XR (2019) miRNA-451a regulates RPE function through promoting mitochondrial function in proliferative diabetic retinopathy. Am J Physiol Endocrinol Metab 316(3):E443. https://doi.org/10.1152/ajpendo.00360.2018
Stockwell BR (2019) A powerful cell-protection system prevents cell death by ferroptosis. Nature 575(7784):597–598. https://doi.org/10.1038/d41586-019-03145-8
Sun Y, Zheng Y, Wang C, Liu Y (2018a) Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis 9(7):753. .https://doi.org/10.1038/s41419-018-0794-4
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W (2018b) Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 17(1):147. https://doi.org/10.1186/s12943-018-0897-7
Ting DS, Tan KA, Phua V, Tan GS, Wong CW, Wong TY (2016) Biomarkers of Diabetic Retinopathy. Curr Diab Rep 16(12):125. https://doi.org/10.1007/s11892-016-0812-9
Totsuka K, Ueta T, Uchida T, Roggia MF, Nakagawa S, Vavvas DG, Honjo M, Aihara M (2019) Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp Eye Res 181:316–324. https://doi.org/10.1016/j.exer.2018.08.019
van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O’Toole SA, Rasko JE, Holst J (2016) ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35(24):3201–3208. https://doi.org/10.1038/onc.2015.381
Wang XN, Li ST, Li W, Hua YJ, Wu Q (2018a) The thickness and volume of the choroid, outer retinal layers and retinal pigment epithelium layer changes in patients with diabetic retinopathy. Int J Ophthalmol 11(12):1957–1962. https://doi.org/10.18240/ijo.2018a.12.14
Wang S, Li L, Chen X, Huang X, Liu J, Sun X, Zhang Y, Shen T, Guo J, Man Y, Tang W, Dou L, Li J (2018b) miR–338–3p mediates gluconeogenesis via targeting of PP4R1 in hepatocytes. Mol Med Rep 18(4):4129–4137. https://doi.org/10.3892/mmr.2018b.9400
Wang H, Tao Y (2019) Choroidal structural changes correlate with severity of diabetic retinopathy in diabetes mellitus. BMC Ophthalmol 19(1):186. https://doi.org/10.1186/s12886-019-1189-8
Wang L, Liu Y, Zhao TL, Li ZZ, He JY, Zhang BJ, Du HZ, Jiang JW, Yuan ST, Sun L (2019a) Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine 57:117–128. https://doi.org/10.1016/j.phymed.2018.12.011
Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, Wang X, Zhou H, Cao Y, Liu S, Yan Q, Tao Y, Zhang B (2019b) Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ 26(11):2329–2343. https://doi.org/10.1038/s41418-019-0304-y
Wei H, Cao C, Wei X, Meng M, Wu B, Meng L, Wei X, Gu S, Li H (2020) Circular RNA circVEGFC accelerates high glucose-induced vascular endothelial cells apoptosis through miR-338-3p /HIF-1α/VEGFA axis. Aging (Albany NY), 12(14): p. 14365–14375.https://doi.org/10.18632/aging.103478
Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X (2019) Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572(7769):402–406. https://doi.org/10.1038/s41586-019-1426-6
Yang Y, Ishak Gabra MB, Hanse EA, Lowman XH, Tran TQ, Li H, Milman N, Liu J, Reid MA, Locasale JW, Gil Z, Kong M (2019) MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun 10(1):809. https://doi.org/10.1038/s41467-019-08759-0
Yin L, Sun Z, Ren Q, Su X, Zhang D (2019a) Long Non-Coding RNA BANCR Is Overexpressed in Patients with Diabetic Retinopathy and Promotes Apoptosis of Retinal Pigment Epithelial Cells. Med Sci Monit 25:2845–2851. https://doi.org/10.12659/msm.913359
Yin N, Zhu L, Ding L, Yuan J, Du L, Pan M, Xue F, Xiao H (2019b) MiR-135-5p promotes osteoblast differentiation by targeting HIF1AN in MC3T3-E1 cells. Cell Mol Biol Lett 24:51. https://doi.org/10.1186/s11658-019-0177-6
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, Kim JK, Heo Y, Lee HS, Lee MY, Lee CW, Kang JS, Kim YH, Lee J, Choi J, Hwang GS, Bang S, Han JM (2020) A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab 31(2):267–283. https://doi.org/10.1016/j.cmet.2019.11.020
Youngblood H, Robinson R, Sharma A, Sharma S (2019) Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int J Mol Sci 20(19). https://doi.org/10.3390/ijms20194755
Yu Y, Yan R, Chen X, Sun T, Yan J (2020) Paeonol suppresses the effect of ox-LDL on mice vascular endothelial cells by regulating miR-338-3p/TET2 axis in atherosclerosis. Mol Cell Biochem 475(1–2):127–135. https://doi.org/10.1007/s11010-020-03865-w
Yumnamcha T, Devi TS, Singh LP (2019) Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front Neurosci 13:1065. https://doi.org/10.3389/fnins.2019.01065
Zhang Y, Ren S, Yuan F, Zhang K, Fan Y, Zheng S, Gao Z, Zhao J, Mu T, Zhao S, Shang A, Li X, Jie Y (2018) miR-135 promotes proliferation and stemness of oesophageal squamous cell carcinoma by targeting RERG. Artif Cells Nanomed Biotechnol 46(sup2):1210–1219. https://doi.org/10.1080/21691401.2018.1483379
Zhang Y, Xi X, Mei Y, Zhao X, Zhou L, Ma M, Liu S, Zha X, Yang Y (2019) High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed Pharmacother 111:1315–1325. https://doi.org/10.1016/j.biopha.2019.01.034
Zhang Z, Liu R, Shuai Y, Huang Y, Jin R, Wang X, Luo J (2020) ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br J Cancer 122(1):82–93. https://doi.org/10.1038/s41416-019-0637-9
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Dr. Jing Zhou was responsible for the conception of this paper, and also conducted most of the investigation and drafted the manuscript. Dr. Caoyu Sun and Xu Dong provided technical supports, and helped to collect, analyze and visualize the data. Dr. Hui Wang proofread the manuscript, and was responsible for manuscript submission. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee Affiliated to the 4th People’s Hospital of Shenyang.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, J., Sun, C., Dong, X. et al. A novel miR-338-3p/SLC1A5 axis reprograms retinal pigment epithelium to increases its resistance to high glucose-induced cell ferroptosis. J Mol Histol 53, 561–571 (2022). https://doi.org/10.1007/s10735-022-10070-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10070-0