Abstract
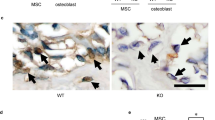
Periodontal ligament-associated protein-1 (PLAP-1) is an osteogenisis negative regulatory gene that inhibits the differentiation of rat bone marrow stromal cells (rBMSCs) into osteoblasts in vitro. The aim of this study was to verify whether PLAP-1 acted as an inhibitory factor of rBMSCs in rat critical-size skull defect repair in vivo. Six-week old male Wistar rats treated with a drill-hole injury were randomly assigned into five groups PLAP-1-transfected rBMSC group, empty vector-transfected rBMSC group, normal rBMSC group, collagen group and blank control group according to the treatment factors. Skull specimens were obtained at 8 weeks after surgery. X-ray examination, histological observation of hard tissue slices (HE, Masson staining and von Kossa staining), immunohistochemical staining were applied to evaluate the repair effects. X-ray examination showed that repair effect of the PLAP-1-transfected rBMSC group was significantly worse than that of the empty vector-transfected rBMSC group and normal rBMSC group. In HE staining, regenerated bone could only be observed in the partial defect area of the PLAP-1-transfected rBMSC group. However, new bone filled nearly the entire defects in the empty vector-transfected rBMSC group and normal rBMSC group. Mineralization of new bone in the two groups was significantly higher than that of the PLAP-1-transfected rBMSC group, according to Masson and von Kossa staining. Meanwhile, the transfected PLAP-1 gene worked well in vivo. Positive expression of PLAP-1 protein was only distributed in the newly formed bone of the PLAP-1-transfected rBMSC group, no positive staining was observed in the other four groups. Overexpression of PLAP-1 in rBMSCs inhibits new bone formation and mineralization in rat critical-size skull defects in vivo.







Similar content being viewed by others
References
Ameye L, Young MF (2002) Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12(9):107R–116R
Chandar N, Donehower L, Lanciloti N (2000) Reduction in p53 gene dosage diminishes differentiation capacity of osteoblasts. Anticancer Res 20(4):2553–2559
Chen S, Young MF, Chakravarti S, Birk DE (2014) Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix Biol 35:103–111
Haÿ E, Nouraud A, Marie PJ (2009) N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing wnt, ERK and PI3 K/Akt signalling. PLoS One 14(12):82–84
Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growthfactor beta. Biochem J 302(Pt 2):527–534
Huang W, Carlsen B, Rudkin G, Berry M, Ishida K, Yamaguchi DT, Miller TA (2004) Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone 34:799–808
Huang K, Kang X, Wang X, Wu S, Xiao J, Li Z, Wu X, Zhang W (2015) Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol Med Rep 11(3):1685–1692
Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA (1996) Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem 317(pt1):59–64
Ikegawa S (2008) Expression, regulation and function of asporin, a susceptibility gene in common bone and joint diseases. Curr Med Chem 15(7):724–728
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar G, Ajita J, Rhee Y, Kim CH, Lim SK (2012) miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. Bone Miner Res 27(8):1669–1679
Kou I, Nakajima M, Ikegawa S (2007) Expression and regulation of the osteoarthritis-associated protein asporin. J Biol Chem 282(44):32193–32199
Li S, Tu Q, Zhang J, Stein G, Lian J, Yang PS et al (2008) Systemically transplanted bone marrow stromal cells contributing to bone tissue regeneration. J Cell Physiol 215:204–209
Lv L, Wang Y, Zhang J, Zhang T, Li S (2014) Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J Mol Histol 45(3):311–320
Nagashima M, Sakai A, Uchida S, Tanaka S, Tanaka M, Nakamura T (2005) Bisphosphonate (YM529) delays the repair of cortical bone defect after drill-hole injury by reducing terminaldifferentiation of osteoblasts in the mouse femur. Bone 36(3):502–511
Nakajima M, Kizawa H, Saitoh M, Kou I, Miyazono K, Ikegawa S (2007) Mechanisms for asporin function and regulation in articular cartilage. Biol Chem 282:32185–32192
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J (2008) Haploinsufficiency of Runx2 results in one formation decrease and different BSPexpression pattern changes in two transgenic mouse models. J Cell Physiol 217(1):40–47
Satoru Y, Masahiro K, Shinya M (2008) PLAP-1: a novel molecule regulating homeostasis of periodontal tissues. Japanese Dent Sci Rev 44:137–144
Sun J, Zhang T, Zhang P, Lv L, Wang Y, Zhang J, Li S (2014) Overexpression of the PLAP-1 gene inhibits the differentiation of BMSCs into osteoblast-like cells. J Mol Histol 45(5):599–608
Tomoeda M, Yamada S, Shirai H, Ozawa Y, Yanagita M, Murakami S (2008) PLAP-1/asporin inhibits activation of BMP receptor via its leucine-rich repeat motif. Biochem Biophys Res Commun 371(2):191–196
von Marschall Z, Fisher LW (2010) Decorin is processed by three isoforms of bonemorphogenetic protein-1 (BMP1). Biochem Biophys Res Commun 391(3):1374–1378
Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B (2006) p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 172(1):115–125
Wang Y, Lv L, Yu X, Zhang T, Li S (2014) The characteristics of epithelial cell rests of Malassez during tooth eruption of development mice. J Mol Histol 45(1):1–10
Yamada S, Murakami S, Matoba R, Ozawa Y, Yokokoji T, Nakahira Y, Ikezawa K, Takayama S, Matsubara K, Okada H (2001) Expression profile of active genes inhuman periodontal ligament and isolation of PLAP-1, a novel SLRP family gene. Gene 275(2):279–286
Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, Ikegawa S, Saito M, Toyosawa S, Murakami S (2007) PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem 282(32):23070–23080
Yu XJ, Botchuey EA, Levine EM, Pollack SR, Laurencin CT (2004) Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. PNAS 101:11203–11208
Yu X, Lv L, Zhang J, Zhang T, Xiao C, Li S (2015) Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J Mol Histol 46(2):195–203
Zhang PP, Li S, Yang PS, Sun J (2010) Construction and confirmation of a recombinant eukaryotic expression plasmid pBABE-hygro-PLAP-1. Shanghai Kou Qiang Yi Xue 19:635–640
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81271138). Open Foundation of Shandong Provincial Key Laboratory of Oral Biomedicine (SDKQ201403).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xijiao Yu and Jing Sun have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, X., Sun, J., Hu, Y. et al. Overexpression of PLAP-1 in bone marrow stromal cells inhibits the rat critical-size skull defect repair. J Mol Hist 46, 251–261 (2015). https://doi.org/10.1007/s10735-015-9623-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9623-6