Abstract
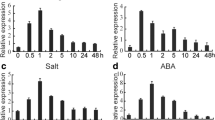
Protein dephosphorylation mediated by serine/threonine protein phosphatase 2A (PP2A) plays critical roles in regulation of abiotic stress responses in plants. Here, we report the involvement of a B″ regulatory subunit of PP2A in wheat (Triticum aestivum L.), TaPP2AbB″-γ, in the positive regulation of stress responses. The transcript of TaPP2AbB″-γ was induced in roots of wheat seedlings by salinity, drought, cold stresses and exogenous ABA application. Overexpression of TaPP2AbB″-γ enhanced the tolerance to various environmental stresses in transgenic Arabidopsis seedlings. Yeast two-hybrid assay demonstrated that TaPP2AbB″-γ interact with the first 100 amino acids of TaBZR1, a positive regulator of Brassinosteroids (BRs) signaling. The expression of TaBZR1 gene was up-regulated in roots upon exposure to NaCl stress. All our results suggested that TaPP2AbB″-γ functions in improving abiotic stress tolerance by directly binding to TaBZR1, pointing to a possible cross-talk of BR signaling with TaPP2AbB″-γ mediated stress response.








Similar content being viewed by others
References
Blakeslee JJ, Zhou HW, Heath JT, Skottke KR, Barrios JA, Liu SY, DeLong A (2008) Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots. Plant Physiol 146:539–553
Clough SJ, Bent AF (2010) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Delong A (2006) Switching the flip: protein phosphatase roles in signaling pathways. Curr Opin Plant Biol 9:470–477
Eichhorn PJ, Creyghton MP, Bernards R (2009) Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta 1795:1–15
Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C (2007) Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci 12:169–176
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Hasegawa PM, Bressan RA (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Ihsan MZ, El-Nakhlawy FS, Ismail SM, Shah F, Ihsanullah D (2016) Wheat phenological development and growth studies as affected by drought and late season high temperature stress under arid environment. Front Plant Sci 7:795
Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353:417–439
Janssens V, Jordens J, Stevens I, Van Hoof C, Martens E, De Smedt H, Engelborghs Y, Waelkens E, Goris J (2003) Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B”/PR72 subunit of protein phosphatase 2A. J Biol Chem 278:10697–10706
Jia J, Zhao S, Kong X, Li Y, Zhao G, He W, Appels R, Pfeifer M, Tao Y, Zhang X (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704
Kirik A, Ehrhardt DW, Kirik V (2012) TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells. Plant Cell 24:1158–1170
Koyama ML, Levesley A, Koebner RMD, Flowers TJ, Yeo AR (2001) Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol 125:406–422
Kuhn BM, Nodzyński T, Errafi S, Bucher R, Gupta S, Aryal B, Dobrev P, Bigler L, Geisler M, Zažímalová E (2017) Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. Sci Rep 7:41906
Lechward K, Awotunde OS, Swiatek W, Muszyńska G (2001) Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim Pol 48:921–933
Liu SH, Wang CX, Mao XG, Liu HM, Li A, Jing RL (2010) Cloning of protein phosphatase 2A regulatory subunit gene TaBβ-1 and its expression patterns under abiotic stresses in wheat. Sci Agric Sin 385:778–792
Liu D, Li A, Mao X, Jing R (2014) Cloning and characterization of TaPP2AbB”-α, a member of the PP2A regulatory subunit in wheat. PLoS ONE 9:e94430
Mujeeb-Kazi A, Leon JLDD (2002) Conventional and alien genetic diversity for salt tolerant wheats: focus on current status and new germplasm development. Springer, Amsterdam
Pais SM, Gonzalez MA, Tellez-Inon MT, Capiati DA (2009) Characterization of potato (Solanum tuberosum) and tomato (Solanum lycopersicum) protein phosphatases type 2A catalytic subunits and their involvement in stress responses. Planta 230:13–25
Pang X, Mao X, Jing R, Shi J, Gao T, Chang X, Li Y (2007) Analysis of gene expression profile responsed to water stress in wheat Triticum aestivum L. seedling. Acta Agron Sin 32:333–336
Rubbo SD, Irani NG, Russinova E (2011) PP2A phosphatases: the “on-off” regulatory switches of brassinosteroid signaling. Sci Signal 4:pe25
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Slupe AM, Merrill RA, Strack S (2011) Determinants for substrate specificity of protein phosphatase 2A. Enzyme Res 2011:398751
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng Z, Gampala SS, Gendron JM, Jonassen EM, Lillo C, DeLong A, Burlingame AL, Sun Y, Wang ZY (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13:124–131
Wright AJ, Gallagher K, Smith LG (2009) discordia1 and alternative discordia1 function redundantly at the cortical division site to promote preprophase band formation and orient division planes in maize. Plant Cell 21:234–247
Xu C, Jing R, Mao X, Jia X, Chang X (2007) A wheat (Triticum aestivum) protein phosphatase 2A catalytic subunit gene provides enhanced drought tolerance in tobacco. Ann Bot 99:439–450
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565
Zhao L, Wang L, Chi C, Lan W, Su Y (2017) The emerging roles of phosphatases in Hedgehog pathway. Cell Commun Signal 15:35
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFD0100605, 2017YFD0300202).
Author information
Authors and Affiliations
Contributions
RJ and DL conceived and designed the experiments. DL and BL performed the most experiments and analyzed the data. GF performed the yeast two-hybrid assays. XM and AL performed the experiments of protoplast transfection and fluorescence microscopy analyses. XC prepared the plant materials. DL and RJ contributed to the writing and revising of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The funding bodies had no role in study design, data collection, analyses and interpretation, decision to publish or preparation of manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, D., Li, B., Feng, G. et al. TaPP2AbBʺ-γ, a wheat regulatory subunit of PP2A enhanced abiotic stress tolerance. Plant Growth Regul 89, 345–355 (2019). https://doi.org/10.1007/s10725-019-00540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00540-z