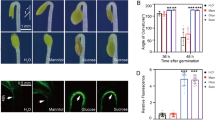
Abstract
Sorghum bicolor produces the cyanogenic glucoside dhurrin, a secondary metabolite integral to plant defence and stress responses. Dhurrin production is both developmentally and environmentally regulated in S. bicolor, with high levels of variation within and between lines. Such phenotypic variation may result from polymorphic differences or epigenetic modifications in genes associated with cyanogenesis. In this study the chemical 5-Azacytidine was used to assess S. bicolor’s response to genome-wide demethylation, which had not previously been investigated in the context of cyanogenic glucoside regulation. Morphological changes, the expression levels of key genes involved in dhurrin synthesis and turnover, and the cyanogenic potential (HCNp) of leaf tissues were analysed. Treatment resulted in alterations in dhurrin synthesis, gene expression, and dhurrin levels, suggesting that DNA methylation is involved in the regulation of HCNp in the initial stages of S. bicolor development. Previously identified EMS mutants from the adult cyanide deficient class (acdc) have been found to exhibit altered dhurrin concentrations during development. This study shows that acdc mutants possess a CΔT change in the promoter of CYP79A1, a key gene in dhurrin synthesis, and that this mutation is stably inherited and associated with the acdc phenotype. To further investigate the role of epigenesis in dhurrin production, we determine the methylation status of the 250 bp region surrounding the CΔT mutation site in wild-type and mutant plants over two stages of development.








Similar content being viewed by others
Abbreviations
- 5-azaC:
-
5-Azacytidine
- CNglc:
-
Cyanogenic glucoside
- dpg:
-
Days post germination
- EMS:
-
Ethyl methanesulfonate
- HCNp:
-
Hydrogen cyanide potential
References
Allen G, Flores-Vergara M, Krasynanski S, Kumar S, Thompson W (2006) A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325
Amoah S et al (2012) A hypomethylated population of Brassica rapa for forward and reverse epi-genetics. BMC Plant Biol 12:193
Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from Cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin: cloning, functional expression in pichia pastoris, and substrate specificity of the isolated recombinant enzymes. J Biol Chem 275:1966–1975
Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450. CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36(1):393–405 CYP71E .
Ballhorn DJ, Kautz S, Laumann JM (2016) Herbivore damage induces a transgenerational increase of cyanogenesis in wild lima bean (Phaseolus lunatus). Chemoecology 26:1–5
Baulcombe DC, Dean C (2014) Epigenetic regulation in plant responses to the environment. Cold Spring Harb Perspec Biol. https://doi.org/10.1101/cshperspect.a019471
Bjarnholt et al (2018) Glutathione transferases catalyze recycling of auto-toxic cyanogenic glucosides in sorghum. Plant J 94:1109–1125. https://doi.org/10.1111/tpj.13923
Blomstedt CK et al (2012) A combined biochemical screen and TILLING approach identifies mutations in Sorghum bicolor L. Moench resulting in acyanogenic forage production. Plant Biotechnol J 10:54–66. https://doi.org/10.1111/j.1467-7652.2011.00646.x
Blomstedt CK, Rosati VC, Lindberg Møller B, Gleadow R (2018) Counting the costs: nitrogen partitioning in Sorghum mutants. Funct Plant Biol. https://doi.org/10.1071/FP17227
Burke JJ, Payton P, Chen J, Xin Z, Burow G, Hayes C (2015) Metabolic responses of two contrasting sorghums to water-deficit stress. Crop Sci 55:344–353
Busk PK, Møller BL (2002) Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol 129:1222–1231. https://doi.org/10.1104/pp.000687
Christman JK (2002) 5-Azacytidine and 5-aza-2 [variant prime]-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21:5483
Chu HY, Wegel E, Osbourn A (2011) From hormones to secondary metabolism: the emergence of metabolic gene clusters in plants. Plant J 66:66–79
Colinas M, Goossens A (2018) Combinatorial transcriptional control of plant specialized metabolism. Trends Plant Sci 23:324–326
Darbani B, Motawia MS, Olsen CE, Nour-Eldin HH, Møller BL, Rook F (2016) The biosynthetic gene cluster for the cyanogenic glucoside dhurrin in Sorghum bicolor contains its co-expressed vacuolar MATE transporter. Sci Rep 6:37079. https://doi.org/10.1038/srep37079
Fieldes M, Schaeffer S, Krech M, Brown J (2005) DNA hypomethylation in 5-azacytidine-induced early-flowering lines of flax. Theor Appl Genet 111:136–149
Finnegan EJ, Peacock WJ, Dennis ES (1996) Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci USA 93:8449–8454
Finnegan EJ, Peacock WJ, Dennis ES (2000) DNA methylation, a key regulator of plant development and other processes. Curr Opin Genet Dev 10:217–223
Getachew G, Putnam DH, De Ben CM, De Peters EJ (2016) Potential of sorghum as an alternative to corn forage. Am J Plant Sci 7:1106–1121
Gleadow RM, Møller BL (2014) Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol 65:155–185 doi. https://doi.org/10.1146/annurev-arplant-050213-040027
Gleadow RM, Bjarnholt N, Jørgensen K, Fox J, Miller RM (2012) Detection, identification and quantitative measurement of cyanogenic glycosides. In: Narwal SS, Szajdak L, Sampietro DA (eds) Research methods in plant science: soil allelochemicals, vol. 1. International Allelopathy Foundation, Studium Press, Houston, pp. 283–310
Gruenbaum Y, Naveh-Many T, Cedar H, Razin A (1981) Sequence specificity of methylation in higher plant DNA. Nature 292(5826):860
Halkier BA, Møller BL (1989) Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol 90:1552–1559
Henry IM et al (2014) Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell 26:1382–1397. https://doi.org/10.1105/tpc.113.121590
Jenrich R, Trompetter I, Bak S, Olsen CE, Møller BL, Piotrowski M (2007) Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc Natl Acad Sci USA 104:18848–18853. https://doi.org/10.1073/pnas.0709315104
Joel AJ (2013) Epigenetic responses to drought stress in rice (Oryza sativa L.). Physiol Mol Biol Plants 19:379–387
Jones DA (1998) Why are so many food plants cyanogenic? Phytochemistry 47:155–162
Jørgensen K et al (2011) Biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in cassava: isolation, biochemical characterization, and expression pattern of CYP71E7, the oxime-metabolizing cytochrome P450 enzyme. Plant Physiol 155:282–292. https://doi.org/10.1104/pp.110.164053
Kahn RA, Bak S, Svendsen I, Halkier BA, Møller BL (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115:1661–1670
Kannangara R, Motawia MS, Hansen NKK, Paquette SM, Olsen CE, Møller BL, Jørgensen K (2011) Characterization and expression profile of two UDP-glucosyltransferases, UGT85K4 and UGT85K5, catalyzing the last step in cyanogenic glucoside biosynthesis in cassava. Plant J 68:287–301. https://doi.org/10.1111/j.1365-313X.2011.04695.x
Kellenberger RT, Schlüter PM, Schiestl FP (2016) Herbivore-induced DNA demethylation changes floral signalling and attractiveness to pollinators in Brassica rapa. PLoS ONE 11:e0166646. https://doi.org/10.1371/journal.pone.0166646
Kim M, Ohr H, Lee JW, Hyun Y, Fischer RL, Choi Y (2008) Temporal and spatial downregulation of Arabidopsis MET1 activity results in global DNA hypomethylation and developmental defects. Mol Cells 26:611
Kou HP et al (2011) Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.). J Plant Physiol 168:1685–1693. https://doi.org/10.1016/j.jplph.2011.03.017
Laursen T et al (2016) Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354:890–893
Marshall O (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20:2471–2472
Miller RE, Gleadow R, Cavagnaro TR (2013) Age versus stage: does ontogeny modify the effect of phosphorus and arbuscular mycorrhizas on above-and below-ground defence in forage sorghum? Plant Cell Environ 37:929–942
Neilson EH, Edwards A, Blomstedt C, Berger B, Møller BL, Gleadow R (2015) Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. J Exp Bot 66(7):1817–1832
Nielsen LJ et al (2016) Dhurrin metabolism in the developing grain of Sorghum bicolor (L.) Moench investigated by metabolite profiling and novel clustering analyses of time-resolved transcriptomic data. BMC Genom 17:1021
Nützmann H-W, Osbourn A (2014) Gene clustering in plant specialized metabolism. Curr Opin Biotechnol 26:91–99
O’Donnell NH, Møller BL, Neale AD, Hamill JD, Blomstedt CK, Gleadow RM (2013) Effects of PEG-induced osmotic stress on growth and dhurrin levels of forage sorghum. Plant Physiol Biochem 73:83–92
Olson A, Klein RR, Dugas DV, Lu Z, Regulski M, Klein PE, Ware D (2014) Expanding and vetting Sorghum bicolor gene annotations through transcriptome and methylome sequencing. Plant Gen 7:1–20. https://doi.org/10.3835/plantgenome2013.08.0025
Pandey G, Sharma N, Sahu PP, Prasad M (2016) Chromatin-based epigenetic regulation of plant abiotic stress response. Curr Genom 17:490–498. https://doi.org/10.2174/1389202917666160520103914
Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10:11
Sano H, Kamada I, Youssefian S, Katsumi M, Wabiko H (1990) A single treatment of rice seedlings with 5-azacytidine induces heritable dwarfism and undermethylation of genomic DNA. Mol Gen Genet 220:441–447
Saze H (2008) Epigenetic memory transmission through mitosis and meiosis in plants. In: Seminars in cell & developmental biology vol 6. Elsevier, Amsterdam, pp. 527–536
Sharma R, Vishal P, Kaul S, Dhar MK (2017) Epiallelic changes in known stress-responsive genes under extreme drought conditions in Brassica juncea (L.) Czern. Plant Cell Rep 36:203–217
Simon P (2003) Q-gene: processing quantitative real-time RT–PCR data. Bioinformatics 19:1439–1440
Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, Raghothama KG, Meagher RB (2010) Histone H2A. Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol 152:217–225
Takos AM et al (2011) Genomic clustering of cyanogenic glucoside biosynthetic genes aids their identification in Lotus japonicus and suggests the repeated evolution of this chemical defence pathway. Plant J 68:273–286. https://doi.org/10.1111/j.1365-313X.2011.04685.x
Turco GM et al (2017) DNA methylation and gene expression regulation associated with vascularization in Sorghum bicolor. New Phytol 214:1213–1229
Wang W-S et al (2011) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62(6):1951–1960. https://doi.org/10.1093/jxb/erq391
Acknowledgements
We thank Dr Alan Neale for valuable comments on the manuscript and Dr Peter Stuart (Seed Tek Pty Ltd) for carrying out the crosses between the various sorghum lines analysed in this study. The project was supported by Australian Research Council Grant Nos. LP100100434 and DP130101049 to RG. VCR is supported by an Australian Government Research Training Program Scholarship and AW Howard Memorial Trust Inc. Research Fellowship.
Author information
Authors and Affiliations
Contributions
Experimental studies and analyses were carried out by VCR, AAQ, SMF and CKB. RG contributed to the design and coordination of the studies, as well as data interpretation. VCR, RG and CKB drafted the manuscript. All authors contributed to the approval of the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rosati, V.C., Quinn, A.A., Fromhold, S.M. et al. Investigation into the role of DNA methylation in cyanogenesis in sorghum (Sorghum bicolor L. Moench). Plant Growth Regul 88, 73–85 (2019). https://doi.org/10.1007/s10725-019-00489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00489-z