Abstract
Both cDNA including 5′UTR and 3′UTR and genomic clones of cinnamyl alcohol dehydrogenase (CAD) were isolated and characterized from a pulp-yielding leguminous tree Leucaena leucocephala (LlCAD1). The deduced amino acid sequence shared high identity with orthologous sequences of Acacia mangium × Acacia auriculiformis (83%), Medicago sativa (83%), Nicotiana tabaccum (83%) and Aralia cordata (81%). Full length cDNA contained 78 bases of 5′UTR and 283 bases of 3′UTR, while the genomic clone contained 5 exons and 4 introns. Western blot analysis revealed elevated expression of LlCAD1 in seedling roots and shoots compared to leaves. Sense and antisense CAD tobacco transgenics showed increased and reduced CAD activity accompanied by a change in monomeric lignin composition. Histochemical staining of lignin in down-regulated plants suggested an increase in aldehyde units and a decrease in S/G ratio. Down-regulation of CAD resulted in accumulation of syringic, ferulic, p-coumaric and sinapic acids compared to untransformed controls. These observations were validated by anatomical studies of down-regulated transgenic stems which showed thin walled, elongated phloem and xylem fibres, accompanied by a reduction in the density of vessel elements and amount of secondary xylem when compared to untransformed plants. Furthermore, Klason lignin analysis of CAD antisense transgenics showed 7–32% reduced lignin and normal phenotype as compared to untransformed plants. Such a reduction was not noticed in up-regulated transgenics. These results demonstrate a unique opportunity to explore the significant role that down-regulation of CAD gene plays in reducing lignin content thereby offering potential benefits to the pulp and paper industry.








Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Baucher M, Chabbert B, Pilate G, Van Doorsselaere J, Tollier MT, Petit-Conil M, Cornu D, Monties B, Van Montagu M, Inze D, Jouanin L, Boerjan W (1996) Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol 112:1479–1490
Berlyn GP, Miksche JP, Sass JE (1976) Botanical microtechnique and cytochemistry. The Iowa State University Press, Ames
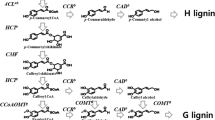
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Boudet AM, Grima-Pettenati J (1996) Lignin genetic engineering. Mol Breed 2:25–39
Boudet AM, Lapierre C, Grima-Pettenati J (1995) Biochemistry and molecular biology of lignification. New Phytol 129:203–236
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brill EM, Abrahams S, Hayes CM, Jenkins CL, Watson JM (1999) Molecular characterization and expression of a wound-induced cDNA encoding a novel cinnamoyl alcohol dehydrogenase enzyme in lucerne (Medicago sativa L.). Plant Mol Biol 41:279–291
Bucholtz DL, Cantrell RP, Axtell JD, Lechtenberg VL (1980) Lignin biochemistry of normal and brown midrib mutant sorghum. J Agric Food Chem 28:1239
Campbell MM, Sederoff RR (1996) Variation in lignin content and composition. Mechanism of control and implications for the genetic improvement of plants. Plant Physiol 110:3–13
Chabannes M, Abdellah B, Catherine L (2001) Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J 28:257–270
Chabbert B, Tollier MT, Monties B (1993) Expression of brown midrib mutations on grass lignin. In: Proceedings of the 38th congress on lignin. Japan Wood Research Society, Kagawa, pp 33–36
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Dean JFD, Eriksson KEL (1992) Biotechnological modifications of lignin structure and composition in forest trees. Holzforschung 46:135–147
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length c DNA from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA 85:8998–9002
Galliano H, Heller W, Sandermann HJ (1993) Ozone induction and purification of spruce cinnamoyl alcohol dehydrogenase. Phytochemistry 32:557–563
Goffner D, Joffroy I, Grima-Pettenati J, Halpin C, Knight ME, Schuch W, Boudet AM (1992) Purification and characterization of isoforms of cinnamoyl alcohol dehydrogenase from Eucalyptus xylem. Planta 188:48–53
Grand C, Sarni F, Boudet AM (1985) Inhibition of cinnamyl alcohol dehydrogenase activity and lignin synthesis in poplar (Populus × euramericana Dode) tissues by two organic compounds. Planta 163:232–237
Grisebach H (1981) Lignins. In: Conn EE (ed) Secondary plant products (The Biochemistry of Plants, A Comprehensive Treatise), vol 7. Academic Press, New York, pp 457–478
Halpin C, Knight ME, Geoffrey AF, Malcolm MC, Boudet AM, Boon JJ, Chabbert B, Marie-Thérèse T, Schuch W (1994) Manipulation of lignin quality by down-regulation of cinnamyl alcohol dehydrogenase. Plant J 6:339–350
Hibino T, Keiji T, Tetsu K, Daisuke S, Takayoshi H (1995) Increase of cinnamaldehyde groups in lignin of transgenic tobacco plants carrying an antisense gene for cinnamyl alcohol dehydrogenase. Biosci Biotechnol Biochem 59:929–931
Higuchi T (1985) Biosynthesis of lignin. In: Higuchi T (ed) Biosynthesis and biodegradation of wood components. Academic Press, Orlando, pp 141–160
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hu WJ, Popko JL, Lung JR (1998) Transgenic aspen trees with reduced lignin quantity and increased cellulose content (Abstract 011), presented at the Anslem Payen award symposium, 1998, Spring National American Chemical Society meeting, March 29–April 2, Dallas, Texas, USA
Iiyama K, Pant R (1988) The mechanism of the Maule colour reaction. Introduction of methylated syringyl nuclei into soft wood lignin. Wood Sci Technol 22:167–175
Jorgenson LR (1931) Brown mid rib in maize and its linkage relations. J Am Soc Agron 23:549–557
Jung HG, Weiting N (1998) Lignification of plant cell walls: impact of genetic manipulation. Proc Natl Acad Sci USA 95:12742–12743
Kajita S, Katayama Y, Omori S (1996) Alterations in the biosynthesis of lignin in transgenic plants with chimeric genes for 4-coumarat: coenzyme A ligase. Plant Cell Physiol 117:761–770
Kajita S, Hishiyama S, Tomimura Y, Katayama Y, Omori S (1997) Structural characterization of modified lignin in transgenic tobacco plants in which the activity of 4-cumarate:coenzyme A ligase is depressed. Plant Physiol 114:871–879
Kim H, Ralph J, Yahiaoui N, Pean M, Boudet AM (2000) Cross-coupling of hydroxycinnamyl aldehydes into lignins. Org Lett 2:2197–2200
Kolattukudy PE (1981) Structure, biosynthesis and biodegradation of cutin and suberin. Annu Rev Plant Physiol 32:539–567
Krik TK, Obst JR (1988) Lignin determination. Methods Enzymol 161:87–101
Kuc J, Nelson OE (1964) The abnormal lignins produced by the brown- mid rib mutants of maize. Arch Biochem Biophys 105:103–113
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41:455–496
Lynn DG, Chang M (1990) Phenolic signals in cohabitation: implication for plant development. Annu Rev Plant Physiol Plant Mol Biol 41:497–526
Mac Kay J, O’Malley D, Sederoff R (1995) A null mutation in CAD (cinnamyl alcohol dehydrogenase) and its effect in phenolic metabolism in pine. IUFRO, Ghent, pp 26–30
Meyermans H, Morreel K, Lapierre C, Brigitte P, André DB, Busson R, Herdewijn P, Devreese B, Beeumen JV, Marita JM, Ralph J, Chen C, Burggraeve B, Van Montagu M, Messens E, Boerjan W (2000) Modifications in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme A D-methoxy transferase, an enzyme involved in lignin biosynthesis. J Biol Chem 275:36899–36909
O’Malley DM, Porter S, Sederoff RR (1992) Purification, characterization and cloning of cinnamyl alcohol dehydrogenase in loblolly pine (Pinus taeda L.). Plant Physiol 98:1364–1371
Pearson W, Lipman D (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Pillonel C, Mulder MM, Boon JJ, Forster B, Binder A (1991) Involvement of cinnamyl alcohol dehydrogenase in the control of lignin formation in Sorghum bicolor L. Planta 185:538–544
Pillonel C, Hunziker P, Binder A (1992) Multiple forms of the constitutive wheat cinnamyl alcohol dehydrogenase. J Exp Bot 43:299–305
Piquemal J, Lapierre C, Myton K, O’Connell A, Schuch W, Grima-Pettenati J, Boudet AM (1998) Down-regulation of cinnamoyl CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J 13:71–83
Prashant S, Sunita MSL, Pramod S, Gupta RK, Kumar SA, Karumanchi SR, Rawal SK, Kavi Kishor PB (2011) Down-regulation of Leucaena leucocephala cinnamoyl CoA reductase (LlCCR) gene induces significant changes in phenotype, soluble phenolic pools and lignin in transgenic tobacco. Plant Cell Rep 30:2215–2231. doi:10.1007/s00299-011-1127-6
Ralph J, Hatfield RO, Piquemal J, Yahiaoui N, Pean M, Lapierre C, Boudet AM (1998) NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnmylalcohol dehydrogenase and cinnamoyl Co-A reductase. Proc Natl Acad Sci USA 95:12803–12808
Rao KS, Rajput KS (2001) Xylem structure and annual rhythm of development in the twigs of Acacia nilotica (L.) Del. growing in different forests of Gujarat state (India). Phyton 41:1–12
Saka S (1991) Chemical composition and distribution. In: David N-SH, Shiraishi N (eds) Wood and cellulosic chemistry. Marcel Dekker Inc., New York, pp 59–88
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schreiber L (1996) Chemical composition of casparian strips isolated from Clivia miniata Reg. roots: evidence for lignin. Planta 199:596–601
Sekhar PN, Kumar RD, Sirisha VL, Prashant S, Kavi Kishor PB (2009) Phylogenetic analysis, homology modelling, molecular dynamics, docking and structure based designing new inhibitors against cinnamyl alcohol dehydrogenase (CAD) cloned and sequenced from a leguminous tree subabul (Leucaena leucocephala). OJB 11:103–124
Sewalt VJH, Ni W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA (1997) Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of l-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol 115:41–50
Speer EO (1987) A method of retaining phloroglucinol proof of lignin. Stain Technol 62:279–280
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Umezawa T, Davin LB, Lewis NG (1991) Formation of lignans (−) secoisolariciresinol and (−) matairesinol with Forsythia intermedia cell—free extracts. J Biol Chem 266:10210–10217
Van Blockland R, De Lange P, Mol JNM, Kooter JM (1993) Modulation of gene expression in plants by antisense genes. In: Lebleu B (ed) Antisense research and applications. CRC Press, Boca Raton, pp 125–148
Van Doorsselaere J, Baucher M, Chognot E, Chabbert B, Tollier MT, Petit-Conil M, Leple J-C, Pilate G, Cornu D, Monties B, Van Montagu M, Inze D, Boerjan W, Jouanin L (1995) A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid)-methyltransferase activity. Plant J 8:855–864
Vignols F, Rigau J, Torres MA, Capellades M, Puigdom`enech P (1995) The brown mid rib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid)-methyltransferase. Plant Cell 7:407–416
Watson CF, Grierson R (1993) Antisense RNA in plants. In: Hiatt A (ed) Transgenic plants: fundamentals and applications. Marcel Dekker Inc., New York, pp 255–281
Wyrambik D, Grisebach H (1975) Purification and properties of isoenzymes of cinnamyl alcohol dehydrogenase from soybean cell suspension cultures. Eur J Biochem 59:9–15
Yahiaoui N, Marque C, Myton KE, Negrel J, Boudet AM (1998) Impact of different levels of cinnamyl alcohol dehydrogenase down-regulation on lignins of transgenic tobacco plants. Planta 204:8–15
Acknowledgments
This work was supported by a grant from the Council of Scientific and Industrial Research (CSIR-NMITLI), New Delhi, India and we gratefully acknowledge the financial assistance. We are also thankful to the UGC, New Delhi for financial assistance in the form of Centre for Advanced Studies to the Department of Genetics, and DBT-OU-ISLARE program to the Osmania University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sirisha, V.L., Prashant, S., Ranadheer Kumar, D. et al. Cloning, characterization and impact of up- and down-regulating subabul cinnamyl alcohol dehydrogenase (CAD) gene on plant growth and lignin profiles in transgenic tobacco. Plant Growth Regul 66, 239–253 (2012). https://doi.org/10.1007/s10725-011-9647-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9647-1