Abstract
Key message
Arabidopsisˈs HsfA1d was found to confer resistance to pea plant against heat stress by enhancing the activity of antioxidant enzymes and decreasing hydrogen per oxide compared to wild type plants.
Abstract
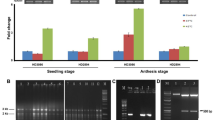
Pea is one of the important cool season food crops. Heat is the most critical abiotic stress that adversely affects pea growth and economic yield. Response to heat stress in plants is mostly regulated by heat shock factors (HSFs). The current study aimed at improving heat tolerance of pea plant by incorporating heat shock factor, HsfA1d, isolated from Arabidopsis thaliana. Gateway cloning strategy was used for cloning of HsfA1d in plant expression vector pGWB415. The target gene was introduced into pea plant using Agrobacterium-mediated transformation approach. Transformant pea plants revealed five fold increase in the expression of HsfA1d under heat stress (42 °C) compared to room temperature (25 °C). Transgenic and wild type plants exhibited significant difference in their H2O2 content, under thermal stress. Upon exposure to heat stress, wild type plants shown significant increase in their H2O2 content while no such elevation was found in transgenic plants. Similarly, significant difference in superoxide dismutase activities of transgenic and wild type plants was recorded, under thermal stress. Heat stress induced more pronounced increase in SOD activities of transgenic plants compared to wild type. Highly significant increase in proline content and ascorbate peroxidase activities of transgenic plants was observed upon exposure to thermal stress.





Similar content being viewed by others
References
Ali G, Hadi F, Ali Z, Tariq M, Khan MA (2007) Callus induction and in vitro complete plant regeneration of different cultivars of tobacco (Nicotiana tabacum L.) on media of different hormonal concentrations. Biotechnology 6(4):561–566
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water- stress studies. Plant Soil 39(1):205–207
Bokszczanin KL, Fragkostefanakis S, Bostan H, Bovy A, Chaturvedi P, Chiusano ML, Firon N, Iannacone R, Jegadeesan S, Klaczynskid K, Li H (2013) Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front Plant Sci 4:315
El-Esawi MA, Alayafi AA (2019) Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 10(1):56
Froger A, Hall JE (2007) Transformation of plasmid DNA into E. coli using the heat shock method. J Vis Exp 6:e253. https://doi.org/10.3791/253
Guilioni L, Wery J, Lecoeur J (2003) High temperature and water deficit may reduce seed number in the pea field purely by decreasing plant growth rate. Funct Plant Biol 30(11):1151–1164
Gupta NK, Agarwal S, Agarwal VP, Nathawat NS, Gupta S, Singh G (2013) Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol Plant 35(6):1837–1842
Ilse W, Serge R, Rony S, László S (2000) Irreversible heat inactivation of DNase I without RNA degradation. BioTechniques 29(2):252–256
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Mashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium mediated tumifaciens. Nat Biotechnol 14(6):745–750
Ismail A, Tiong NW, Tan ST, Azlan A (2009) Antioxidant properties of selected non-leafy vegetables. Nutr Food Sci 39(2):176–180
Jasani HV, Umretiya NG, Kapuria MN, Dharajiya DT, Khatrani TJ, Pagi NK, Parmar LD (2016) In vitro regeneration of pigeonpea [Cajanus cajan (L.) Millsp.] genotype GT 101 using cotyledonary node. Indian J Sci Technol. 9(45):1–5
Jiang Y, Lahlali R, Karunakaran C, Kumar S, Davis AR, Bueckert RA (2015) Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ 38(11):2387–2397
Karimi M, Depicker A, Hilson P (2007) Recombinational cloning with plant gateway vectors. Plant Physiol 145(4):1144–1154
Kavitah G, Thagipour F, Huyop F (2010) Investigation of factors in optimizing Agrobacterium mediated gene transfer in Citrullus lanatus cv round dragon. J Biol Sci 10(3):209–216
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10(3):310–316
Ludwig-Muller J, Vertocnik A, Town CD (2005) Analysis of indole-3-butyric acid- induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 56(418):2095–2105
Mostofa MG, Yoshida N, Fujita M (2014) Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul 73(1):31–44
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Naz N, Durrani F, Shah Z, Khan NA, Ullah I (2019) Influence of heat stress on growth and physiological activities of potato (Solanum tuberosum L.). Phyton 87:225–230
Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S (2006) Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J 48(4):535–547
Panchuk II, Volkoy RA, Schollf F (2002) Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol 129(2):838–853
Qian J, Chen J, Liu YF, Yang LL, Li WP, Zhang LM (2014) Overexpression of Arabidopsis HsfA1a enhances diverse stress tolerance by promoting stress-induced Hsp expression. Genet Mol Res 13(1):1233–1243
Qiu XB, Shao YM, Miao S, Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63(22):2560–2570
Ridge PE, Pye DL (1985) The effects of temperature and frost at flowering on the yield of peas grown in a Mediterranean environment. Field Crops Res 12:339–346
Scharf KD, Berberich T, Ebersberger I (1819) Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochimica et Biophysica Acta (BBA) Gene Regul Mech 2:104–119
Shah Z, Shah SH, Jan A, Ali GS (2017) Overexpression of the heat shock-specific transcription factor HSFA1D enhances thermotolerance on tobacco plants. Sarhad J Agric 33(1):162–170
Shah Z, Shah SH, Ali GS, Munir I, Khan RS, Iqbal A, Ahmed N, Jan A (2020) Introduction of Arabidopsis’s heat shock factor HsfA1d mitigates adverse effects of heat stress on potato (Solanum tuberosum L.) plant. Cell Stress Chaperones 2:1–7
Trinidad TP, Mallillin AC, Loyola AS, Sagum RS, Encabo RR (2010) The potential health benefits of legumes as a good source of dietary fibre. Br J Nutr 103(4):569–574
Xue Y, Peng R, Xiong A, Li X, Zha D, Yao Q (2010) Over-expression of heat shock protein gene hsp26 in Arabidopsis thaliana enhances heat tolerance. Biol Plant 54(1):105–111
Xu Q, Xu X, Shi Y, Xu J, Huang B (2014) Transgenic tobacco plants overexpressing a grass PpEXP1 gene exhibit enhanced tolerance to heat stress. PLoS ONE 9(7):e100792. https://doi.org/10.1371/journal.pone.0100792
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252
Acknowledgements
The present work was financially supported by Higher Education Commission Islamabad Pakistan. The research facilities were extended by University of Science and Technology Bannu Pakistan.
Author information
Authors and Affiliations
Contributions
ZS conducted the main experiment and collected data. AI helped in conducting research. FUK helped in data analysis. HUK checked the manuscript time to time for improvement. FD contributed in manuscript writing. MZA helped in graph setting.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, Z., Iqbal, A., Khan, F.U. et al. Genetic manipulation of pea (Pisum sativum L.) with Arabidopsisˈs heat shock factor HsfA1d improves ROS scavenging system to confront thermal stress. Genet Resour Crop Evol 67, 2119–2127 (2020). https://doi.org/10.1007/s10722-020-00966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-020-00966-9