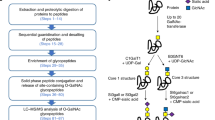
Abstract
In this work O-linked glycopeptides bearing mucin core-1 type structures were enriched from human serum. Since about 70 % of the O-glycans in human serum bind to the plant lectin Jacalin, we tested a previously successful protocol that combined Jacalin affinity enrichment on the protein- and peptide-level with ERLIC chromatography as a further enrichment step in between, to eliminate the high background of unmodified peptides. In parallel, we developed a simpler and significantly faster new workflow that used two lectins sequentially: wheat germ agglutinin and then Jacalin. The first lectin provides general glycopeptide enrichment, while the second specifically enriches O-linked glycopeptides with Galβ1-3GalNAcα structures. Mass spectrometric analysis of enriched samples showed that the new sample preparation method is more selective and sensitive than the former. Altogether, 52 unique glycosylation sites in 20 proteins were identified in this study.


Similar content being viewed by others
References
Helenius, A., Aebi, M.: Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 (2004)
Varki, A.: Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3, 97–130 (1993)
Haltiwanger, R.S., Lowe, J.B.: Role of glycosylation in development. Annu. Rev. Biochem. 73, 491–537 (2004)
Rudd, P.M., Elliott, T., Cresswell, P., Wilson, I.A., Dwek, R.A.: Glycosylation and the immune system. Science 291, 2370–2376 (2001)
Brockhausen, I.: Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 (2006)
Arnold, J.N., Saldova, R., Hamid, U.M., Rudd, P.M.: Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 8, 3284–3293 (2008)
Grigorian, A., Mkhikian, H., Li, C.F., Newton, B.L., Zhou, R.W., Demetriou, M.: Pathogenesis of multiple sclerosis via environmental and genetic dysregulation of Nglycosylation. Semin. Immunopathol. 34, 415–424 (2012)
Christiansen, M.N., Chik, J., Lee, L., Anugraham, M., Abrahams, J.L., Packer, N.H.: Cell surface protein glycosylation in cancer. Proteomics 14, 525–546 (2013)
Scott, D.W., Patel, R.P.: Endothelial heterogeneity and adhesion molecules Nglycosylation: implications in leukocyte trafficking in inflammation. Glycobiology 23, 622–633 (2013)
Stuchlova Horynova, M., Raska, M., Clausen, H., Novak, J.: Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell. Mol. Life Sci. 70, 829–839 (2013)
Venkatakrishnan, V., Packer, N.H., Thaysen-Andersen, M.: Host mucin glycosylation plays a role in bacterial adhesion in lungs of individuals with cystic fibrosis. Expert Rev. Respir. Med. 7, 553–576 (2013)
Schedin-Weiss, S., Winblad, B., Tjernberg, L.O.: The role of protein glycosylation in Alzheimer disease. FEBS J. 281, 46–62 (2014)
Hägglund, P., Matthiesen, R., Elortza, F., Højrup, P., Roepstorff, P., Jensen, O.N., Bunkenborg, J.: An enzymatic deglycosylation scheme enabling identification of core fucosylated N-glycans and O-glycosylation site mapping of human plasma proteins. J. Proteome Res. 6, 3021–3031 (2007)
Zhang, H., Li, X.J., Martin, D.B., Aebersold, R.: Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 21, 660–666 (2003)
Nilsson, J., Rüetschi, U., Halim, A., Hesse, C., Carlsohn, E., Brinkmalm, G., Larson, G.: Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 6, 809–811 (2009)
Halim, A., Nilsson, J., Rüetschi, U., Hesse, C., Larson, G.: Human urinary glycoproteomics; attachment site specific analysis of N- and O-linked glycosylations by CID and ECD. Mol. Cell. Proteomics (2012). doi:10.1074/mcp.M111.013649
Halim, A., Rüetschi, U., Larson, G., Nilsson, J.: LC-MS/MS characterization of O-glycosylation sites and glycan structures of human cerebrospinal fluid glycoproteins. J. Proteome Res. 12, 573–584 (2013)
Steentoft, C., Vakhrushev, S.Y., Vester-Christensen, M.B., Schjoldager, K.T., Kong, Y., Bennett, E.P., Mandel, U., Wandall, H., Levery, S.B., Clausen, H.: Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 (2011)
Vakhrushev, S.Y., Steentoft, C., Vester-Christensen, M.B., Bennett, E.P., Clausen, H., Levery, S.B.: Enhanced mass spectrometric mapping of the human GalNAc-type O-glycoproteome with SimpleCells. Mol. Cell. Proteomics 12, 932–944 (2013)
Steentoft, C., Vakhrushev, S.Y., Joshi, H.J., Kong, Y., Vester-Christensen, M.B., Schjoldager, K.T., Lavrsen, K., Dabelsteen, S., Pedersen, N.B., Marcos-Silva, L., Gupta, R., Bennett, E.P., Mandel, U., Brunak, S., Wandall, H.H., Levery, S.B., Clausen, H.: Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 32, 1478–1488 (2013)
Yang, Z., Halim, A., Narimatsu, Y., Jitendra Joshi, H., Steentoft, C., Schjoldager, K.T., Alder Schulz, M., Sealover, N.R., Kayser, K.J., Paul Bennett, E., Levery, S.B., Vakhrushev, S.Y., Clausen, H.: The GalNAc-type O-Glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol. Cell. Proteomics 13, 3224–3235 (2014)
Campos, D., Freitas, D., Gomes, J., Magalhães, A., Steentoft, C., Gomes, C., Vester-Christensen, M.B., Ferreira, J.A., Afonso, L.P., Santos, L.L., Pinto de Sousa, J., Mandel, U., Clausen, H., Vakhrushev, S.Y., Reis, C.A.: Probing the O-glycoproteome of gastric cancer cell lines for biomarker discovery. Mol. Cell. Proteomics 14, 1616–1629 (2015)
Trinidad, J.C., Schoepfer, R., Burlingame, A.L., Medzihradszky, K.F.: N- and O-glycosylation in the murine synaptosome. Mol. Cell. Proteomics (2013). doi:10.1074/mcp.M113.030007
Medzihradszky, K.F., Kaasik, K., Chalkley, R.J.: Tissue-specific glycosylation at the glycopeptide level. Mol. Cell. Proteomics 14, 2103–2110 (2015)
Darula, Z., Medzihradszky, K.F.: Affinity enrichment and characterization of mucin core-1 type glycopeptides from bovine serum. Mol. Cell. Proteomics 8, 2515–2526 (2009)
Darula, Z., Sherman, J., Medzihradszky, K.F.: How to dig deeper? Improved enrichment methods for mucin core-1 type glycopeptides. Mol. Cell. Proteomics (2012). doi:10.1074/mcp.O111.016774
Darula, Z., Chalkley, R.J., Lynn, A., Baker, P.R., Medzihradszky, K.F.: Improved identification of O-linked glycopeptides from ETD data with optimized scoring for different charge states and cleavage specificities. Amino Acids 41, 321–328 (2011)
Zubarev, R.A., Horn, D.M., Fridriksson, E.K., Kelleher, N.L., Kruger, N.A., Lewis, M.A., Carpenter, B.K., McLafferty, F.W.: Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 72, 563–573 (2000)
Syka, J.E.P., Coon, J.J., Schroeder, M.J., Shabanowitz, J., Hunt, D.F.: Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 101, 9528–9533 (2004)
Good, D.M., Wirtala, M., McAlister, G.C., Coon, J.J.: Performance characteristics of electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 6, 1942–1951 (2007)
Brockhausen, I., Schachter, H., Stanley, P.: O-GalNAc glycans. In: Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E. (eds.) Essentials of Glycobiology. 2nd edition. Chapter 9. Cold Spring Harbor Laboratory Press, Cold Spring Harbor (2009)
Halim, A., Brinkmalm, G., Ruetschi, U., Westman-Brinkmalm, A., Portelius, E., Zetterberg, H., Blennow, K., Larson, G., Nilsson, J.: Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl. Acad. Sci. U. S. A. 108, 11848–11853 (2011)
Bern, M., Kil, Y.J., Becker, C.: Byonic: advanced peptide and protein identification software. Curr. Protoc. Bioinformatics. Chapter 13:Unit13.20 (2012)
Domon, B., Costello, C.E.: A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 5, 397–409 (1988)
Baker, P.R., Trinidad, J.C., Chalkley, R.J.: Modification site localization scoring integrated into a search engine. Mol. Cell. Proteomics (2011). doi:10.1074/mcp.M111.008078
Yamada, K., Hyodo, S., Kinoshita, M., Hayakawa, T., Kakehi, K.: Hyphenated technique for releasing and MALDI MS analysis of O-glycans in mucin-type glycoprotein samples. Anal. Chem. 82, 7436–7443 (2010)
Yabu, M., Korekane, H., Miyamoto, Y.: Precise structural analysis of O-linked oligosaccharides in human serum. Glycobiology 24, 542–553 (2014)
Alpert, A.J.: Electrostatic repulsion hydrophilic interaction chromatography for isocratic separation of charged solutes and selective isolation of phosphopeptides. Anal. Chem. 80, 62–76 (2008)
Wiśniewski, J.R., Zougman, A., Nagaraj, N., Mann, M.: Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009)
Trinidad, J.C., Barkan, D.T., Gulledge, B.F., Thalhammer, A., Sali, A., Schoepfer, R., Burlingame, A.L.: Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics 11, 215–229 (2012)
Medzihradszky, K.F., Kaasik, K., Chalkley, R.J.: Characterizing sialic acid variants at the glycopeptide level. Anal. Chem. 87, 3064–3071 (2015)
Saba, J., Dutta, S., Hemenway, E., Viner, R.: Increasing the productivity of glycopeptides analysis by using higher-energy collision dissociation-accurate mass-product-dependent electron transfer dissociation. Int. J. Proteomics (2012). doi:10.1155/2012/560391
Baker, P.R., Chalkley, R.J.: MS-viewer: a web-based spectral viewer for proteomics results. Mol. Cell. Proteomics 13, 1392–1396 (2014)
Hortin, G.L., Sviridov, D., Anderson, N.L.: High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin. Chem. 54, 1608–1616 (2008)
Hao, P., Guo, T., Sze, S.K.: Simultaneous analysis of proteome, phospho- and glycoproteome of rat kidney tissue with electrostatic repulsion hydrophilic interaction chromatography. PLoS One (2011). doi:10.1371/journal.pone.0016884
de Jong, E.P., Griffin, T.J.: Online nanoscale ERLIC-MS outperforms RPLC-MS for shotgun proteomics in complex mixtures. J. Proteome Res. 11, 5059–5064 (2012)
Hao, P., Ren, Y., Dutta, B., Sze, S.K.: Comparative evaluation of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) and high-pH reversed phase (Hp-RP) chromatography in profiling of rat kidney proteome. J. Proteomics 82, 254–262 (2013)
Li, Q., Jain, M.R., Chen, W., Li, H.: A multidimensional approach to an in-depth proteomics analysis of transcriptional regulators in neuroblastoma cells. J. Neurosci. Methods 216, 118–127 (2013)
Loroch, S., Schommartz, T., Brune, W., Zahedi, R.P., Sickmann, A.: Multidimensional electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) for quantitative analysis of the proteome and phosphoproteome in clinical and biomedical research. Biochim. Biophys. Acta 1854, 460–468 (2015)
Sok Hwee Cheow, E., Hwan Sim, K., de Kleijn, D., Neng Lee, C., Sorokin, V., Sze, S.K.: Simultaneous enrichment of plasma soluble and extracellular vesicular glycoproteins using prolonged ultracentrifugation-ERLIC approach. Mol. Cell. Proteomics 14, 1657–1671 (2015)
Chang, C.F., Pan, J.F., Lin, C.N., Wu, I.L., Wong, C.H., Lin, C.H.: Rapid characterization of sugar-binding specificity by in-solution proximity binding with photosensitizers. Glycobiology 21, 895–902 (2011)
Tachibana, K., Nakamura, S., Wang, H., Iwasaki, H., Tachibana, K., Maebara, K., Cheng, L., Hirabayashi, J., Narimatsu, H.: Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology 16, 46–53 (2006)
Windwarder, M., Yelland, T., Djordjevic, S., Altmann, F.: Detailed characterization of the O-linked glycosylation of the neuropilin-1 c/MAM-domain. Glycoconj. J. (2015)
Acknowledgments
The authors are grateful to Andrew Alpert for the generous gift of the PolyWAX column, Samuel Myers for the WGA-POROS column, ProteinMetrics Inc. for access to the Byonic software. We would like to thank Agnes Arva for technical assistance. We appreciate the help of Robert Chalkley in editing our manuscript. This work was supported by the following grants: National Institutes of Health NIGMS 8P41GM103481, Hungarian Scientific Research Fund 105611 (to Z. Darula), National Research, Development and Innovation Fund BAROSS-DA07-DA-ESZK-07-2008-0036 (to the Biological Research Centre, HAS, director: P. Ormos), New Szechenyi Plan GOP-1.1.1-11-2012-0452, and by the Howard Hughes Medical Institute (to the Bio-Organic Biomedical Mass Spectrometry Resource at UCSF, Director: A. L. Burlingame). Z. Darula was supported by the Janos Bolyai Fellowship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 584 kb)
Rights and permissions
About this article
Cite this article
Darula, Z., Sarnyai, F. & Medzihradszky, K.F. O-glycosylation sites identified from mucin core-1 type glycopeptides from human serum. Glycoconj J 33, 435–445 (2016). https://doi.org/10.1007/s10719-015-9630-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-015-9630-6