Abstract
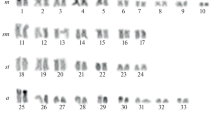
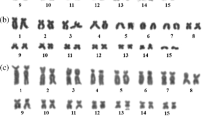
Based on morphological, bioacoustics, and morphological traits, the genus Scinax has been subdivided into two major clades: S. catharinae and S. ruber. The first clade includes S. catharinae and S. perpusillus groups, whereas the second clade includes S. rostratus and S. uruguayus groups. Chromosome morphology, NOR and C-banding patterns of variation support these clades. This study aims the cytogenetic characterization of five species currently included in the S. perpusillus group: Scinax sp. (gr. perpusillus), S. arduous, S. belloni, S. cosenzai, and S. v-signatus, including standard cytogenetic techniques and repetitive DNA FISH probes. All species had 2n = 24 chromosomes. Nucleolar organizing regions occurred in chromosome pair 6 in all species, but differed in their locations among some species, suggesting a putative synaponomastic character for the clade. In S. belloni, the first chromosome pair was a metacentric, contrasting with the submetacentric first pair reported in all other species of the genus. Scinax sp. (gr. perpusillus) and S. v-signatus had similar karyotypic formulae, suggesting they are related species. Scinax cosenzai had a divergent C-banding pattern. Repetitive DNA probes hybridized more frequently in chromosomal subtelomeric regions in all species indicating recent cladogenesis in these species. Karyotypic evidence indicates unreported high levels of stabilization within S. perpusillus and in S. catharinae clade, resulting in a wealth of characters potentially informative for higher phylogenetic analyses.




Similar content being viewed by others
References
Alves-Silva R, Silva HR (2009) Life in bromeliads: reproductive behavior and the monophyly of the Scinax perpusillus species group (Anura: Hylidae). J Nat Hist 43:205–217
Anderson K (1991) Chromosome evolution in Holarctic Hyla tree frogs. In: Green DM, Sessions SK (eds) Amphibian cytogenetics and evolution. Academic Press, San Diego, p 189
Baldissera FA, Oliveira PSL, Kasahara S (1993) Cytogenetics of four Brazilian Hyla species (Amphibia, Anura) and description of a case with a supernumerary chromosome. Brazil J Genet 16:335–345
Barrio A, Pistol De Rubel D (1970) Características del cariotipo de los pséudidos (Amphibian, Anura). Physis 29:505–510
Beçak ML (1968) Chromosomal analysis of eighteen species of Anura. Caryologia 21:191–208
Bogart JP (1973) Evolution of anuran karyotypes. In: Vial JL (ed) Evolutionary biology of the anurans. University of Missouri Press, Columbia, pp 337–349
Bogart JP, Bogart JE (1971) Genetic compatibility experiments between some South American anuran amphibians. Herpetol 27(3):229–235
Busin CS, Lima AP, Prado CPA, Strüssmann C, Siqueira S, Recco-Pimentel MS (2006) Chromosomal differentiation of populations of Lysapsus limellus limellus, L. l. bolivianus, and of Lysapsus caraya (Hylinae, Hylidae). Micron 37:355–362
Cardozo DE, Leme DM, Bortoleto JF, Catroli GF, Baldo D, Faivovich J, Kolenc F, Silva APZ, Borteiro C, Haddad CFB, Kasahara S (2011) Karyotypic data on 28 species of Scinax (Amphibia: Anura: Hylidae) diversity and informative variation. Copeia 2011:251–263
Catroli GF, Kasahara S (2009) Cytogenetic data on species of the family Hylidae (Amphibia, Anura): results and perspectives. Publicatio 15:67–86
Charlesworth B, Sniegowski P, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215–220
Cioffi MB, Martins C, Bertolo LAC (2009) Comparative chromosome mapping of repetitive sequences. Implications for genomic evolution in the fish, Hoplias malabaricus. BMC Genet 10:34
Cioffi MB, Kejnovský E, Bertollo LAC (2010) The chromosomal distribution of microsatellite repeats in the genome of the wolf fish Hoplias malabaricus, focusing on the sex chromosomes. Cytogenet Genome Res 132:289–296
Cioffi MB, Kejnovský E, Marquioni V, Poltronieri J, Molina WF, Diniz D, Bertollo LAC (2012) The key role of repeated DNAs in sex chromosome evolution in two fish species with ZW sex chromosome system. Mol Cytogent 5:28
Duellman WE (1967) Additional studies of chromosomes of anuran amphibians. Syst Zool 16:38–43
Faivovich J (2002) A cladistic analysis of Scinax (Anura: Hylidae). Cladistics 18:367–393
Frost DR (2015) Amphibian species of the world 6.0: an online reference. http://research.amnh.org/herpetology/amphibia/index.html. Accessed in 14 March 2015
Green DM, Sessions SK (eds) (1991) Nomenclature for chromosomes. In: Amphibian cytogenetics and evolution. Academic Press, San Diego, p 223
Gruber SL, Zina J, Narimatsu H, Haddad CFB, Kasahara S (2012) Comparative karyotype analysis and chromosome evolution in the genus Aplastodiscus. BMC Genet 13:28
Howell WM, Black DA (1980) Controlling silver-staining of nucleolus organizer regions with a protective colloidal developer: step method. Experientia 36:1014–1015
Jentzsch IMV, Bagshaw AT, Buschiazzo E, Merkel A, Gemmell NJ (2008) Evolution of microsatellite DNA. eLS 361:1–14
Kasahara S, Silva APZ, Gruber S, Haddad CFB (2003) Comparative cytogenetic analysis on four tree frog species (Anura, Hylidae, Hylinae) from Brazil. Cytogenet Genome Res 103:155–162
King M (1990) Amphibia. In: Jhon B, Gwent C (eds) Animal cytogenetics. Gebruder Borntraeger, Berlin, pp 1–241
Lesk AM (2012) Microarrays and transcriptomics. In: Introduction to genomics, 2nd edn. Oxford University Press, Oxford, pp 265–293
Martins C, Ferreira IA, Oliveira C, Foresti F, Galetti PE (2006) A tandemly repetitive centromeric DNA sequence of the fish Hoplias malabaricus (Characiformes: Erythrinidae) is derived from 5S rDNA. Genetica 127:133–141
Maxon R, Cohn R, Kedes L (1983) Expression and organization of histones genes. Annu Rev Genet 17:239–277
Nogueira L, Paim F, Diniz D, Solé M, Affonso P, Siqueira S, Sampaio I (2015a) Cytogentic analysis of Scinax auratus and Scinax eurydice (Anura, Hylidae) with emphasis on cytotaxonomy. Comp cytogenet 9(2):227–236
Nogueira L, Zanoni JB, Solé M, Affonso P, Siqueira S, Sampaio I (2015b) Cytogenetic studies in six species of Scinax (Anura, Hylidae) clade Scinax ruber from northern and northeastern Brazil. Genet Mol Biol 38(2):156–161
Nunes RRA, Fagundes V (2008) Cariótipos de oito espécies de anfíbios das subfamílias Hylinae e Phyllomedusinae (Anura, Hylidae) do Espírito Santo, Brasil. Bol Mus Biol Mello Leitão 23:21–36
Peixoto OL (1987) Caracterização do grupo ‘‘perpusillus’’ e revalidação da posição taxonômica de Ololygon perpusilla perpusilla e Ololygon perpusilla v-signata (Amphibia, Anura, Hylidae). Arq Univ Fed Rural Rio J 10:37–49
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci 83:2934–2938
Poltronieri J, Marquioni V, Bertollo LAC, Kejnovský E, Molina WF, Liehr T, Cioffi MB (2013) Comparative chromosomal mapping of microsatellites in Leporinus species (Characiformes, Anostomidae): unequal accumulation on the W chromosomes. Cytogenet Genome Res 142:40–45
Pombal JP, Bastos RP (2003) Vocalizações de Scinax perpusillus (A. Lutz & B. Lutz) e S. arduous Peixoto (Anura, Hylidae), com comentários taxonômicos. Rev Bras Zool 20:607–610
Pombal JP, Haddad CFB, Kasahara S (1995) A new species of Scinax (Anura: Hylidae) from southeastern Brazil with comments on the genus. J Herpetol 29:1–6
Rabello MN (1970) Chromosomal studies in Brazilian anurans. Caryologia 23:45–49
Schmid M (1978) Chromosome banding in Amphibia: constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66:361–388
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58:307–324
Stephan W, Walsh B (2007) Repetitive DNA: evolution. eLS 26:1–7
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Acknowledgments
The authors wish to thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Nível Superior (CAPES) for financial support. The Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) issued the collect permits 33456-1 and 26157-7 to JVAL and RNF. Financial support was given to RNF through a CNPq scientific productivity fellowship. Comments of anonymous reviewers helped to improve an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Peixoto, M.A.A., Lacerda, J.V.A., Coelho-Augusto, C. et al. The karyotypes of five species of the Scinax perpusillus group (Amphibia, Anura, Hylidae) of southeastern Brazil show high levels of chromosomal stabilization in this taxon. Genetica 143, 729–739 (2015). https://doi.org/10.1007/s10709-015-9870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-015-9870-1