Abstract
Are acids natural kinds? Or are they merely relevant kinds? Although acidity has been one of the oldest and most important concepts in chemistry, surprisingly little ink has been spilled on the natural kind question. I approach the question from the perspective of microstructural essentialism. After explaining why both Brønsted acids and Lewis acids are considered functional kinds, I address the challenges of multiple realization and multiple determination. Contra Manafu and Hendry, I argue that the stereotypical properties of acids are not multiply realized. Instead, given the equivalence between the proton-donating and electron-accepting mechanisms of Brønsted and Lewis, respectively, I show that acidity as a property type can be identified with a unique microstructural property, namely the presence of a LUMO or other low energy empty orbital. In doing so, I defend the view that the Lewis theory encompasses Brønsted–Lowry, and that all Brønsted acids are also Lewis acids. Contra Hacking and Chang, I thus maintain that the different concepts of acidity do not crosscut, and that the hierarchy requirement is met. Finally, by characterizing natural kinds as powerful objects and by adopting a dispositional view of functions, I illustrate how the microessentialist can make sense of the latent and relational character of most acids. In sum, I contend that acids are genuine natural kinds, even for the microstructural essentialist.


Similar content being viewed by others
Notes
Roughly speaking, a ‘kind’ pertains to the world, whereas a ‘category’ belongs to our language or scientific theories. As Muhammad Ali Khalidi (2013, p. xi) observes, “there is often a close connection between the kinds that are present in the world and the categories that we invent to understand the world”.
Examples include the set of all coffee grinders under € 500, or the group of all European citizens born on a Tuesday.
Natural kinds, then, can be construed as sets or classes of objects that resemble one another by virtue of sharing one or more natural properties. Whether natural kinds are just that (sets of objects), or whether they should be conceived of as universals, is a question that goes beyond the scope of the present paper. Similarly, I will not take a stand in the debate on whether an ontology of natural properties is sufficient to make sense of natural kinds, or whether natural kinds are a sui generis type of entity requiring their own distinct ontological category. On the latter view, a natural kind would be an entity over and above the set of natural properties shared by all members of the kind, together with the laws holding these properties together. See Tobin (2013) for a proper analysis and in-depth examination of either option. Finally, in what follows, I will only focus on the metaphysics of natural kinds; the semantics of natural kind terms will not be discussed. For the latter, see the collection of papers in The Semantics and Metaphysics of Natural Kinds (2010).
See, for example, the work of Dupré (1993).
Notice that the term ‘chemical kind’ may refer (i) to microscopic species, such as atoms, ions and molecules, (ii) to chemical substances (as macroscopic bodies of pure or impure stuff), and (iii) to groups of substances. For example, an isolated gold atom is a chemical kind; but so is a macroscopic sample of gold, and so are the coinage metals in group 11 of the periodic table to which gold belongs. Again, a single molecule of ethanol (CH3CH2OH) is a chemical kind; so is the chemical substance ethanol, and so are the alcohols of which ethanol is just one example (albeit the most famous one). The central question in the natural kind debate is whether these chemical kinds are natural kinds. Do they carve nature at its joints, or do they butcher it randomly and artificially?
Although it would no doubt be interesting, and perhaps even illuminating, to also look at the question from the perspective of cluster kinds (as developed by Richard Boyd’s (1991, 1999) homeostatic property cluster theory) or promiscuous kinds (as developed by John Dupré), I will refrain from doing so in this paper.
Tahko (2015, p. 796) defines natural kind essentialism thus: “There are at least some genuine, mind-independent natural kinds that are defined by their essential properties”. Notice that these essential properties need not be elusive; they can be discovered through scientific investigation. For a contemporary defense of natural kind essentialism and further developments, see Bird (2007), Hawley and Bird (2011), Tahko (2015), and the New Essentialism (or scientific essentialism) of Brian Ellis (2001, 2002, 2009) in which a dispositional view of kind essences is developed.
Two brief remarks about the nature of kind essences according to the New Essentialism of Brian Ellis (2001, 2009): 1. An important aspect of Ellis’s framework is that natural kind essences must be intrinsic properties. 2. According to Ellis, these essential properties are conceived of as powers or dispositional properties. Not everyone agrees with Ellis’s requirements. For example, many advocates of essentialism have dropped the first requirement, arguing that essentialism is compatible with non-intrinsic essential properties. For discussion of the intrinsicness requirement, see Williams (2011), Tahko (2015) and Havstad (2018). I thank Tuomas Tahko for drawing my attention to this, and will return to both of these points in the last two sections of my paper when discussing the latent and relational character of acidity.
Notice that there are various levels of microstructural arrangement. As Tahko (2015, p. 804) points out, the term ‘microstructure’ should be understood as “a placeholder for whatever level of microstructural accuracy one wishes to focus on.” It could be nuclear, atomic, molecular or even supra-molecular.
As I mentioned above, the identification of the substance ‘water’ with the molecular structure ‘H2O’ has been questioned by various philosophers of chemistry. See, for instance, Van Brakel (2000) and Needham (2000, 2002, 2011). More generally, the idea that kind essences are intrinsic and microstructural has faced major criticism. See, for example, LaPorte (2003).
Given this complexity, a further distinction is typically introduced between the primary, secondary, tertiary and quaternary structure of proteins.
Acetic acid (CH3COOH), for example, better known as vinegar in diluted form, is a plant-based substance, and therefore classified as a vegetable acid; hydrochloric acid (HCl) and sulphuric acid (H2SO4), in contrast, are mineral acids.
As Hendry (2006a, p. 866) explains, Lavoisier considered the acids to be a ‘chemical genus’ (in the context of this paper, one could say a ‘chemical kind’) whose characteristic ingredient was the element oxygen, and whose ‘differentiae’ were the different acidifiable ‘principles’, such as nitrogen, sulphur and phosphorous. The more these principles were oxygenated, the stronger the resulting acid. For example, nitric acid (in modern notation, HNO3) is a stronger acid than nitrous acid (HNO2).
Joseph Priestley, it may be added, advanced the idea that carbon dioxide (‘fixed’ or ‘dephlogisticated’ air, as it was called at the time) was the acidifying principle (Finston and Rychtman 1982, p. 2).
The nature of acids and bases has been the subject of continued research and debate for more than three centuries. Both Jensen (1979, chapters 1 & 2) and Finston and Rychtman (1982, chapter 1) provide a historical overview of the evolution of the concept of acidity. See also Walden (1929, part I), Lowry (1936, chapter 2), Luder and Zuffanti (1961, chapter 1), Kauffman (1988), Jensen (2016), Ruthenberg and Chang (2017) and Gerontas (2023) for more on the history of acidity.
Luder and Zuffanti (1961, p. ix), in their classic book on The Electronic Theory of Acids and Bases emphasize that acidity, on the Lewis view, has “nothing to do with the presence of any one element or specific group of elements”, contrary to most of the constitutive views that came before. The authors reiterate their point on p. 2.
Although my focus in this paper will be on the Brønsted–Lowry and Lewis definitions, they are far from the only ‘modern’ definitions of acidity. Even before Brønsted and Lowry, Arrhenius already defined acids as substances that increase the hydrogen ion concentration in water. In analogy with this, the solvent system theory defines acids as solutes that increase the concentration of the cation generated in the autoionization reaction of the protic or aprotic solvent. What is important, for our purposes, is that all these alternate definitions of acidity are also functional definitions.
The relationship between functional kinds and natural kinds is a complicated one, to be further explored in this paper. One worry is that by admitting functional kinds into our ontology, we would seem to end up with a plurality of kind categories. There would be kinds whose essences are microstructural, and other kinds whose essences are functional. This would go against the spirit of natural kind monism, and force us into accepting some form of natural kind pluralism Notice that the monism/pluralism distinction just drawn is not always interpreted in this way. For many, natural kind monism is the thesis that there is only one correct way of carving nature at its joints; natural kind pluralism, then, is the thesis that nature can be carved up in many different, but equally valid ways. On the latter view, objects will oftentimes be cross-classified, depending on our scientific interests. I will return to the problem of crosscutting classifications in Sect. 8.
The multiple realizability thesis originated in the 1970s in discussions about the mind’s relationship to the brain. It was argued that a single mental/psychological kind, such as pain, could be realized by multiple distinct physical kinds, e.g. by different brain states. The thesis was advanced to support antireductionism in philosophy of mind. After all, if the correlation between mental states and brain states is not one-one but one-many, then mental types are not identical to physical types. Mental states do not type-reduce to physical brain states, and so psychophysical reduction must be false, or so the argument went. The multiple realizability thesis, therefore, originally led many philosophers of mind to adopt some form of nonreductive physicalism.
Manafu (2015, p. 48) contrasts the property of being an acid with the property of being an alcohol which, in his opinion, does ‘reduce’ to a microstructural property, namely to the presence of a hydroxyl group in the molecular structure that is bound to a saturated carbon atom. The property of being an alcohol, in other words, is identical (or, at least, co-extensive) with the microstructural property of possessing a hydroxyl group. Notice that this is a type identity theory. The claim, after all, is that all alcohols are hydroxyl-containing compounds.
To put it somewhat differently, according to the multiple realizability thesis, the tokens of a certain type can be realized by the tokens of two or more distinct types (Jaworski 2023). Hence, type-type reduction fails, while token-token reduction still holds true.
According to Manafu, chemistry provides us with many other examples of functional, multiply realized properties (e.g. the property of being a base, a reductant, an oxidant, a metal, etc.). He uses this feature to argue for a novel approach to (ontological) emergence which he calls functional emergence. On this account, it is not the chemical entities, but chemical properties (like acidity), as well as laws and explanations, that emerge from the microphysical level. While these of course depend on the physical level, they do not reduce to it. In that sense, functional emergence is very similar to the position of nonreductive physicalism which also attempts to reconcile our physicalist intuitions that all there is, is ultimately physical with the fact that for some reason or another the special sciences are not reducible to physics. Manafu’s account thus attempts to secure the ontological autonomy of chemistry from physics and also circumvents some of the problems with Hendry’s alternative account of ontological emergence in chemistry, as developed in Hendry (2006b, 2010a, b).
To be fair, while Tahko accepts that biochemical kinds are multiply realized, he believes the challenge of multiple realization can be addressed and ends up defending ontological reductionism about biochemical kinds.
Tahko (2015, p. 804) formulates the microstructuralist thesis as follows: “Necessarily, a sample of a chemical substance A is of the same chemical substance as B if and only if A and B have the same microstructural composition.” Considering the multiple realizability of functional kinds, Kistler (2018) takes issue with this biconditional, much like Manafu and Hendry above. That is, while he accepts the physicalist thesis that if A and B share the same microstructure, then A and B are the same chemical substance, he rejects the reverse microstructuralist thesis that if A and B are the same chemical substance, then they must necessarily have the same microstructure. According to Kistler, then, while the higher-level properties of a chemical substance are obviously not identical to its lower-level properties (contra microstructuralism), they are still ‘nothing over and above’ these lower-level properties (pro physicalism).
A set of properties A is said to supervene upon another set of properties B when there cannot be an A-difference without a B-difference.
For example, while the proton from the hydroxyl group (–OH group) in acetic acid (CH3COOH) is weakly acidic, the three methyl protons are nonacidic. Not all hydrogen-containing compounds, therefore, are necessarily acidic, as Liebig and others before him had assumed.
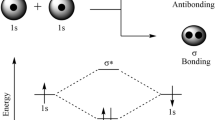
The same can be said for Lewis acids: being an electron pair acceptor is identical to having an empty orbital which is capable of accepting an electron pair from a Lewis base to form an acid–base adduct.
Further on, Manafu (2015, p. 49) reiterates the point that functional properties in chemistry (such as acidity) are multiply realized “in the sense of their being many systems composed of electrons and nuclei that can carry out the specified role”.
As Polger and Shapiro (2016, p. 63) note: “Without reflecting on the issue of relevance, claims of multiple realization become trivial. If variation of any sort at all constitutes multiple realization, the thesis begins to sound more like an a priori commitment than an empirically risky conjecture.” Or again (p. 67): “Variation is everywhere in nature, but multiple realization is not.”
There is a risk that interest-relative considerations may sneak into Polger and Shapiro’s relevance criterion. I do not have the space to discuss this worry here, but see Tahko (2020) for more details.
This is not entirely correct, since presumably one of the criteria for being a corkscrew is that a screw be present to screw into the cork. But this is a microstructural criterion, not a functional one.
Arranging the hydrogen halides in order of increasing acid strength yields the following sequence: HF< HCl< HBr< HI. The reason for this is simple: as one goes down the halogen group in the periodic system, the size of the anion increases. As such, the internuclear distance between the proton and the halogen ion increases, which makes for a weaker bond, and thus a greater proton-releasing ability.
Polger and Shapiro (2016, p. 68) use the same example in The Multiple Realization Book: “isotopes of gold are not different ways of having the atomic number 79 (i.e., being gold)—they are not multiple realizations of gold”.
I am oversimplifying the case here. Not everyone agrees that the isotope effects are chemically irrelevant. VandeWall (2007, p. 918) thus argues that “it remains unclear whether different isotopes of the ‘same element’ belong to the same natural kind.” And Woody and Glymour (2000, p. 24) wonder: “which are the natural kinds, elements, or their isotopes, or both, and why?” But that is an entirely different discussion—one that is of little relevance to our considerations of multiple realization. However, please see Thyssen (2023) for a history of the isotope controversy.
This last statement has to be taken with a grain of salt. According to Ruthenberg (2023), “Thomas Martin Lowry, although a remarkable figure in the history of chemistry of the first half of the 20th century, has not been a co-creator of the modern protonist theory of acidity.” This is in line with a footnote in Bell (1973, p. 4) where the author states: “although Lowry’s paper undoubtedly contains many of the ideas underlying [the proton] definition, especially for bases, it does not contain an explicit definition [...] hence it does not seem justifiable to regard Lowry as one of the originators of the definition.” Where possible, I have thus refrained from using the term ‘Brønsted–Lowry acid’, preferring ‘Brønsted acid’ instead. However, I will continue referring to the ‘Brønsted–Lowry theory/view/definitions’ to highlight the contrast with Lewis.
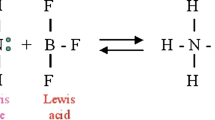
G. N. Lewis (1923, p. 142) wrote: “It seems to me that with complete generality we may say that a basic substance is one which has a lone pair of electrons which may be used to complete the stable group of another atom and that an acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. In other words, the basic substance furnishes a pair of electrons for a chemical bond; the acid substance accepts such a pair.”
Or in the words of Chang (2012, p. 695): “it seems that different acids do their acidic things for different reasons.” Indeed, “It may be the case that there is nothing significant and interesting that is shared in common by all the substances that we classify as acids”, continues Chang (2012, p. 697).
Notice that Putnam (1970, p. 188) answered the question “Are acids natural kinds?” in the affirmative and took ‘acid’ to denote a single natural kind term. But he considered the property of being a proton donor to be the “essential nature” of acids, and thus seems to have been unaware of Lewis’s alternative definition of acids as electron pair acceptors.
Lewis dot formulas are used to graphically represent the outermost valence electrons of chemical species.
A dative bond is a coordinate covalent bond where both bonding electrons are contributed by only one of the two atoms involved in the bond. In other words, dative bonding occurs when one atom donates an electron pair and the other atom accepts the electron pair.
Or in the words of Ellis (2001, p. 56): “if anything is a member of more than one natural kind, then one of these kinds must be a species of the other.” For further discussion of the hierarchy requirement, please see Hacking (1991, 2007), Khalidi (1998, 2013), Ellis (2001, 2002, 2009) and Tobin (2010a, 2012). I should note that Khalidi, Hendry, and Tobin are all highly critical of the hierarchy thesis. Recently, Havstad (2021) has also argued against the “one true taxonomy” visions of natural kindhood.
To cite from yet another author, Norris F. Hall (1940, p. 127) observes that the Lewis system “includes all the acids and bases of the Brønsted system and no other bases, while it points out a host of new acids [...] which the Brønsted system does not recognize as such.” Not surprisingly, Hall includes a diagram much like the one above to illustrate the relationship between the Brønsted–Lowry and Lewis views. According to Luder and Zuffanti (1961, p. 15), finally, the Brønsted–Lowry view, while consistent, is “merely part of the whole picture.”
In another paper, Jensen (2016, p. 4) writes: “the term acid in the Brønsted–Lowry theory refers to the species HB containing both H+ and its conjugate base, whereas in the Lewis theory the term refers only to the H+ portion of HB.” Or, to quote from yet another author, VanderWerf (1961, p. 72–73) writes: “In the Lewis system, hydrogen chloride is not a true acid, since the hydrogen has no available orbital in which to accommodate an additional pair of electrons. But we can conveniently consider hydrogen chloride as a coordinated complex made up of the acid portion proton (H+) and the base portion chloride ion (Cl–). In fact, any potential Brønsted acid HA may be viewed as a coordinated complex, made up of the acid portion H+ and the base portion :A–.” Bell (1969, p. 102), finally, in his book Acids and Bases: Their Quantitative Behaviour, notes that the Lewis scheme “is commonly described as an ‘extension’ of the acid–base concept, but [...] it does in fact involve using the term acid for an essentially different group of substances.”
Utilizing his notion of “epistemic iteration”, Chang (2016, pp. 40–42) identifies 6 different stages in the iterative development of the concept of acidity, “starting with bare sensations [e.g. the sour taste of vegetable acids] and then simple operations [e.g. the color change of indicators] and crude theoretical presumptions [e.g. Lavoisier’s oxygen theory]” to the development of “fully coherent and scientifically accurate” notions of acidity [e.g. the theories of Arrhenius or Brønsted and Lowry].
Lewis initially presented his electronic acid–base definitions in his 1923 book entitled Valence and the Structure of Atoms and Molecules. However, as Jensen (2016, pp. 20–21) remarks, Lewis “did little more than state them, almost as a passing thought, in the middle of a book whose major theme appeared to bear little relation to the subject of acid–base chemistry.” In doing so, Lewis failed to arouse the interest of the broader chemical community, who instead was swayed by the Brønsted–Lowry definitions that had been proposed that same year. It was not until 1938, 15 years later, when Lewis devoted an entire paper to his acid–base definitions—providing necessary examples in support of his electronic view and arguing for its general character—that chemists finally began to pay attention.
Chang seems to have this reaction mechanism in mind when arguing his case that HCl—at least in aqueous solution—is not a Lewis acid. Chang (2012, p.691) thus insists that one is dealing with “conflicting theoretical definitions of acidity.”
Notice that Chang (2012, p. 694) acknowledges this type of reaction mechanism, but only for reactions in gas phase: “if we consider the reaction of HCl in its pure gas phase (not in aqueous solution), for example, with ammonia, we cannot think in terms of the dissociated form of HCl [as in the 2-step picture–P.T.]. The standard explanation in that case seems to be that HCl is a polar molecule, with the electron density heavily distributed around the chlorine nucleus rather than the hydrogen nucleus, allowing the hydrogen end of the molecule to act as an electron-pair acceptor” (emphasis added).
While H+ has an empty 1s orbital and therefore needs a pair of electrons to complete its duet, the boron atom in BF3 has only six electrons in its valence shell, and thus needs an extra electron pair to complete its octet. It may therefore be surprising that SnCl4 is also a Lewis acid. The tin atom in SnCl4, after all, already has a full octet. However, due to the high electronegativity of chlorine, most of the electron density is pulled towards the chlorine atoms, leaving the tin atom with a partial positive charge. Due to this electron deficiency, it can nonetheless attract electrons from Lewis bases, thereby acting as a proper Lewis acid.
A similar definition can be given, mutatis mutandis, for primary bases. Primary Lewis bases can readily donate a pair of electrons. Examples include the ammonia molecule (NH3), the hydroxyl ion (OH–), and the cyanide ion (CN–) ions, among many other Lewis bases.
Lewis further developed his ideas about primary and secondary acids in collaboration with Glenn T. Seaborg, who worked for him as a research associate during the period July 1937—June 1939. Their collaboration on acids and bases led to two publications: Lewis and Seaborg (1939a, 1939b). See also Seaborg (1984) for some personal recollections of his time as research assistant as well as a first-hand account of Lewis’s research style.
Finston and Rychtman (1982, p. 99) acknowledge that the secondary acid category includes “substances that are strictly Lewis acid–base adducts” (viz. the hydrogen acids), but echo Luder in arguing that “[t]here is a basis for this, since the proton does not exist in the free state”. The distinction between primary and secondary acids, I may add, has become obsolete, and is no longer used in modern chemistry.
Although a discussion of the Usanovich, Lapworth–Robinson and Ingold concepts of acids and bases would certainly be interesting, it would make an already long paper even longer. As such, I kindly refer the reader to the discussions in Jensen (1979). See also the illuminating paper in this Special Issue by Flechsig (2023) on the Usanovich definitions.
Jensen herewith echoes Bell (1947, p. 125) who had made the same terminological suggestion to “restrict the term acid to those species covered by the Brønsted–Lowry definition, and to use [...] the term acceptor or acceptor molecule” for the Lewis acids (emphasis in original).
In this respect, continue Finston and Rychtman (1982, p. 62), the Brønsted–Lowry theory “represents almost no advance [...] over concepts formalized nearly a century prior to its appearance.”
In his 1938 paper, Lewis specified four phenomenological criteria for acid–base systems. As Jensen (1979, p. 59) explains, Lewis used these criteria to show that “experimental acidic behavior was not confined to the proton alone, but was exhibited by electron-pair acceptors in general”, and that his electronic acid–base definitions thus “correctly identified [all those] species exhibiting the experimental behavior of acid–base systems”.
The hydrogen ion is unique among cations in having no electrons; it is nothing more than a proton. Due to its small size—its effective radius is about 10–13 cm as compared to 10–8 cm for most other simple ions—it has a very high charge density. As such, free protons are highly unstable, and not capable of independent existence in solution.
All interactions between a Brønsted acid and base can be described by the type reaction: A1 + B2\(\longrightarrow\) B1 + A2, with A1– B1 and A2– B2 two conjugate acid–base pairs. Such reactions are also called protolytic reactions.
According to David D. Lewis (1983, p. 197), while “[a] thing has its intrinsic properties in virtue of the way that thing itself, and nothing else, is”, it has its extrinsic properties in virtue of its interaction with the world.
I omitted the last part of Scerri’s quote for rhetorical reasons. The original read: “It only becomes acidic on reacting with water or another polar solvent”, but the crucial point here is that water (or the other solvent) would thereby act as a base.
Bell (1947, p. 115) notes that in relation to the Arrhenius definition of acids (i.e. substances that produce hydrogen ions in aqueous solution), “it was not clear whether a pure non-conducting substance like anhydrous hydrogen chloride should be called an acid, or whether it became one only in contact with water”. In line with my own view, Bell explains that “it was usually considered that the anhydrous compound was an acid in virtue of its latent tendency to split off hydrogen ions” (emphasis added).
This can be contrasted with categorical properties. A categorical property is a property that, if instantiated by an object, is manifest under all conditions.
The original reads: “Sauren [...] sind Stoffe die einer Abspaltung [...] von Wasserstoffionen fähig sind.”
Scerri, for one, seems to attach some philosophical importance to this fact. After all, in the section on “[s]ome genuine philosophical issues concerning Lewis acidity”, Scerri (2022, p. 401) emphasizes the fact that “a substance is acidic or basic depending on what substance it is chemically related to”.
Water, more precisely, is said to be amphiprotic because it can either gain a proton (to form the hydronium ion H3O+) or lose a proton (to form the hydroxyl ion OH–). Amino acids are another common example of amphiprotic substances due to the presence of both basic and acidic functional groups in their molecular structure, namely the amino group —NH2 and the carboxylic group —COOH.
Nitric acid (HNO3), for example, was formerly known as aqua fortis (or ‘strong water’). Not surprisingly, it is generally considered to be a strong acid. Yet, in the presence of an even stronger acid such as sulphuric acid (H2SO4), HNO3 will act as a base, yielding the nitronium ion (NO2+) after elimination of a water molecule from the protonated nitric acid (H2NO3+) according to the following acid–base reaction: \(\text {HNO}_{3} + 2 \text {H}_{2}\text {SO}_{4} \rightleftharpoons \text {NO}_{2}^{+} + \text {H}_{3}\text {O}^{+} + 2 \text {HSO}_{4}^{-}\). Due to its amphoteric character, HNO3 can even undergo an autoprotolysis reaction, much like water: \(2 \text {HNO}_{3} \rightleftharpoons \text {NO}_{2}^{+} + \text {NO}_{3}^{-} + \text {H}_{2}\text {O}\).
To be clear, Tahko did not have amphoteric substances in mind when he wrote this, but was referring to the work of Tobin (2010b) on moonlighting proteins. Be that as it may, I take Tahko’s views on moonlighting proteins to be directly transferrable to amphoteric substances. Indeed, Tahko (2020, p. 810) himself observes that “amphoteric substances are comparable to functionally promiscuous moonlighting proteins.”
Tahko (2020) develops this dispositional view even further, adopting a powers-based subset strategy to address the challenges of multiple realization and multiple determination. Applying this strategy to the case of amphoterism, the acidic and basic behaviour of an amphoteric substance is to be identified with two distinct sets of causal powers. Importantly, both sets are proper subsets of the larger set of causal powers associated with the microstructure of the amphoteric substance. That is, both sets are realized by the same chemical substance; both the acidic and basic (higher-level) functions are thus included in the (lower-level) causal profile, thereby accounting for the phenomenon of multiple determination.
References
Atkins, P. et al.: Shriver & Atkins’ Inorganic Chemistry, 5th edn. Oxford University Press, Oxford (2010)
Bartol, J.: Biochemical Kinds. Br. J. Philos. Sci. 67, 531–551 (2016)
Bell, R.P.: The use of the terms “Acid” and “Base”. Q. Rev. Chem. Soc. 1, 113–125 (1947)
Bell, R.P.: Acids and Bases: Their Quantitative Behaviour. Methuen and Co, London (1969)
Bell, R. P.: The Proton in Chemistry, 2nd edn. The Baker Series in Chemistry. Cornell University Press, Ithaca, NY (1973)
Bird, A.: Nature’s Metaphysics: Laws and Properties. Clarendon Press, Oxford (2007)
Bjerrum, J.: Die Entwicklungsgeschichte des Säure-Basenbegriffes und über die Zweckmäßigkeit der Einführung eines besonderen Antibasenbegriffes neben dem Säurebegriff [The History of the Development of the Acid-Base Concept and on the Advisability of Introducing a Special Antibase Concept alongside the Acid Concept]. Naturwissenschaften 38, 461–464 (1951)
Boyd, R.: Realism, anti-foundationalism and the enthusiasm for natural kinds. Philos. Stud. 61, 127–148 (1991)
Boyd, R.: Homeostasis, species, and higher taxa. In: Wilson, R. (ed.) Species: New Interdisciplinary Essays, pp. 141–186. MIT Press, Cambridge, MA (1999)
Bronsted, J. N.: Einige Bemerkungen über den Begriff der Säuren und Basen [Some Observations about the Concept of Acids and Bases]. Recueil des Travaux Chimiques des Pays-Bas. 42(8), 718–728 (1923). https://doi.org/10.1002/recl.19230420815
Brzović, Z.: Natural kinds. In: Internet Encyclopedia of Philosophy (2023)
Chakravartty, A.: A Metaphysics for Scientific Realism: Knowing the Unobservable. Cambridge University Press, New York (2007)
Chang, H.: Acidity: the persistence of the everyday in the scientific. Philosophy of Science. 79, 690–700 (2012)
Chang, H.: The rising of chemical natural kinds through epistemic iteration. In: Kendig, C. (ed.) Natural Kinds and Classification in Scientific Practice. Chap. 2, pp. 33–46. Routledge, Abingdon (2016)
Day, M.C., Selbin, J.: Theoretical Inorganic Chemistry. Reinhold Publishing Corporation, NewYork, NY (1962)
Dupré, J.: The Disorder of Things: Metaphysical Foundations of the Disunity of Science. Harvard University Press, Cambridge, MA (1993)
Ellis, B.: Scientific Essentialism. Cambridge University Press, Cambridge (2001)
Ellis, B.: The Philosophy of Nature: A Guide to the New Essentialism. Acumen, Chesham (2002)
Ellis, B.: The Metaphysics of Scientific Realism. Acumen, Durham (2009)
Finston, H.L., Rychtman, A.C.: A New View of Current Acid-Base Theories. Wiley, New York (1982)
Flechsig, G.-U.: Usanovich and his all-in-one acid-base concept. Found. Chem. (2023)
Funkhouser, E.: Multiple Realizability. Philosophy Compass. 2(2), 303–315 (2007)
Gerontas, A.: Acidity: An early history of the concept. Found. Chem. (2023)
Hacking, I.: Representing and Intervening. Cambridge University Press, Cambridge (1983)
Hacking, I.: A tradition of natural kinds. Philosophical Studies. 61(1/2), 109–126 (1991)
Hacking, I.: Natural kinds: Rosy dawn, scholastic twilight. R. Inst. Philos. Suppl. 61, 203–239 (2007). https://doi.org/10.1017/S1358246100009802
Hall, N.F.: Systems of acids and bases. J. Chem. Educ. 17(3), 124–128 (1940)
Havstad, J.C.: Messy chemical kinds. Br. J. Philos. Sci. 69, 719–743 (2018)
Havstad, J.C.: Complexity begets crosscutting, dooms hierarchy (another paper on natural kinds). Synthese 198, 7665–7696 (2021). https://doi.org/10.1007/s11229-020-02539-w
Hawley, K., Bird, A.: What are natural kinds? Philosophical Perspectives. 25: Metaphysics, 205–221 (2011)
Hendry, R.F.: Elements, compounds, and other chemical kinds. Philos. Sci. 73, 864–875 (2006a). https://doi.org/10.1086/518745
Hendry, R.F.: Is there downward causation in chemistry? In: Baird, D., Scerri, E., McIntyre, L. (eds.) Philosophy of Chemistry: Synthesis of a New Discipline, pp. 173–189. Springer, Dordrecht (2006b)
Hendry, R.F.: Microstructuralism: Problems and Prospects. In: Ruthenberg, K., Van Brakel, J. (eds.) Stuff: The Nature of Chemical Substances, pp. 107–120. Königshausen und von Neumann, Würzburg (2008)
Hendry, R.F.: Chemistry: emergence vs. reduction. In: Macdonald, C., Macdonald, G. (eds.) Emergence in Mind, pp. 205–221. Oxford University Press, Oxford (2010a)
Hendry, R.F.: Ontological reduction and molecular structure. Stud. Hist. Philos. Mod. Phys. 41, 183–191 (2010b)
Hendry, R.F.: Chemical substances and the limits of pluralism. Found. Chem. 14, 55–68 (2012)
Hendry, R.F.: Are chemical kinds natural kinds? In: Mäki, U., et al. (eds.) Recent Developments in the Philosophy of Science: EPSA13 Helsinki, European Studies in Philosophy of Science, pp. 251–261. Springer, Cham, Switzerland (2015). https://doi.org/10.1007/978-3-319-23015-3_19
Hendry, R.F.: Natural kinds in chemistry. In: Scerri, E., Fisher, G. (eds.) Essays in the Philosophy of Chemistry. Oxford University Press, New York, NY. Chap. 12, pp. 253–275 (2016)
Jaworski, W.: Mind and multiple realizability. In: Internet Encyclopedia of Philosophy (2023)
Jensen, W.B.: Lewis acid-base theory I: historical development. Chemistry 47(3), 11–14 (1974)
Jensen, W.B.: The Lewis acid-base definitions: a status report! Chem. Rev. 78, 1–22 (1978)
Jensen, W.B.: The Lewis Acid-Base Concepts: An Overview. John Wiley and Sons, New York (1979)
Jensen, W.B.: Acid-base chemistry and related topics, vol. 3. Collected Papers. Oesper Collections, Cincinnati, OH (2016)
Kauffman, G.B.: The Brønsted-Lowry acid-base concept. J. Chem. Educ. 65(1), 28–31 (1988)
Khalidi, M.A.: Natural kinds and crosscutting categories. J. Philos. 95(1), 33–50 (1998)
Khalidi, M.A.: Natural Categories and Human Kinds: Classification in the Natural and Social Sciences. Cambridge University Press, Cambridge (2013)
Kistler, M.: Espèces naturelles, profil causal et constitution multiple. Lato Sensu 3(1), 17–30 (2016)
Kistler, M.: Natural kinds, causal profile and multiple constitution. Metaphysica 19(1), 1–23 (2018). https://doi.org/10.1515/mp-2018-0006
Kolthoff, I.M.: The Lewis and the Brønsted-Lowry definitions of acids and bases. J. Phys. Chem. 48(1), 51–57 (1944). https://doi.org/10.1021/j150433a006
Kolthoff, I.M., Elving, P.J.: Treatise on Analytical Chemistry, vol. 1, 2nd edn. John Wiley and Sons, New York, NY (1978)
Kripke, S.A.: Naming and Necessity. Harvard University Press, Cambridge, MA (1980)
LaPorte, J.: Natural Kinds and Conceptual Change. Cambridge University Press, Cambridge (2003)
Lewis, D.: Extrinsic properties. Philos. Stud. 44(2), 197–200 (1983)
Lewis, G.N.: Valence and the Structure of Atoms and Molecules. American Chemical Society Monograph Series. Chemical Catalog Company, New York, NY (1923)
Lewis, G.N.: Acids and bases. J. Frankl. Inst. 226(3), 293–313 (1938). https://doi.org/10.1016/S0016-0032(38)91691-6
Lewis, G.N., Seaborg, G.T.: Primary and secondary acids and bases. J. Am. Chem. Soc. 61(7), 1886–1894 (1939a). https://doi.org/10.1021/ja01876a068
Lewis, G.N., Seaborg, G.T.: Trinitrotriphenylmethide ion as a secondary and primary base. J. Am. Chem. Soc. 61(7), 1894–1900 (1939b). https://doi.org/10.1021/ja01876a069
Lowry, T.M.: The uniqueness of hydrogen. J. Soc. Chem. Ind. 42(3), 43–47 (1923). https://doi.org/10.1002/jctb.5000420302
Lowry, T.M.: Historical Introduction To Chemistry. MacMillan and Co, London (1936)
Luder, W.F.: The electronic theory of acids and bases. Chem. Rev. 27(3), 547–583 (1940)
Luder, W.F.: Proton-donors in the electronic theory of acids and bases. J. Chem. Educ. 22(6), 301–304 (1945)
Luder, W.F.: Contemporary acid-base theory. J. Chem. Educ. 25(10), 555–558 (1948)
Luder, W.F.: Brønsted and Lewis acidity. J. Chem. Phys. 20, 525 (1952)
Luder, W.F., Zuffanti, S.: The Electronic Theory of Acids and Bases, 2nd edn. Dover Publications, New York (1961)
Manafu, A.: The historical development of the theories of acidity. In: Book of Abstracts for the Summer Symposium of the International Society for the Philosophy of Chemistry (ISPC). Leuven (2012)
Manafu, A.: Concepts of emergence in chemistry. In: Llored, J.-P. (ed.) The Philosophy of Chemistry: Practices, Methodologies, and Concepts. Cambridge Scholars Publishing, Newcastle upon Tyne (2013)
Manafu, A.: How much philosophy in the philosophy of chemistry? J. Gen. Philos. Sci. 45, 33–44 (2014). https://doi.org/10.1007/s10838-014-9267-3
Manafu, A.: A novel approach to emergence in chemistry. In: Scerri, E., McIntyre, L. (eds.) Philosophy of Chemistry: Growth of a New Discipline. Boston Studies in the Philosophy and History of Science, Chap. 4, pp. 39–55. Springer, Dordrecht (2015). https://doi.org/10.1007/978-94-017-9364-3_4
Needham, P.: What is water? Analysis 60, 13–21 (2000)
Needham, P.: The discovery that water is H2O. Int. Stud. Philos. Sci. 16, 205–226 (2002)
Needham, P.: Microessentialism: What is the argument? Noûs 45, 1–21 (2011)
Polger, T.W., Shapiro, L.A.: The Multiple Realization Book. Oxford University Press, Oxford (2016)
Putnam, H.: Is semantics possible? Metaphilosophy 1(3), 187–201 (1970)
Putnam, H.: The meaning of 'meaning'. Minn. Stud. Philos. Sci. 7, 215–271 (1975)
Ruthenberg, K.: Why Lowry? In: Book of Abstracts for the Centenary Workshop on the Bifurcation of Acidity (Protonism vs. Electronism) (2023)
Ruthenberg, K., Chang, H.: Acidity: modes of characterization and quantification. Stud. History Philos. Sci. 65–66, 121–131 (2017)
Ruthenberg, K., Mets, A.: Chemistry is pluralistic. Found. Chem. 22, 403–419 (2020). https://doi.org/10.1007/s10698-020-09378-0
Scerri, E.R.: Hasok Chang on the nature of acids. Found. Chem. 24, 389–404 (2022)
Seaborg, G.T.: The research style of Gilbert N. Lewis. J. Chem. Educ. 61(2), 93–100 (1984). https://doi.org/10.1021/ed061p93
Sidgwick, N.V.: Electronic Theory of Valency. Oxford University Press, New York, NY (1927)
Smart, J.J.C.: The mind/brain identity theory. In: Stanford Encyclopedia of Philosophy (2007)
Stanford, K., Kitcher, P.: Refining the causal theory of reference for natural kind terms. Philos. Stud. Int. J. Philos. Anal. Tradit. 97(1), 99–129 (2000)
Tahko, T.E.: Natural kind essentialism revisited. Mind 124(495), 795–822 (2015). https://doi.org/10.1093/mind/fzv027
Tahko, T.E.: Where do you get your protein? or: biochemical realization. Br. J. Philos. Sci. 71, 799–825 (2020). https://doi.org/10.1093/bjps/axy044
Tantillo, D.J., Seeman, J.I.: On a unified theory of acids and bases: Hasok Chang, Eric R. Scerri, modern theoretical chemistry, and the philosophy of chemistry. Found. Chem., 1–22 (2023)
The Semantics and Metaphysics of Natural Kinds. Routledge, Abingdon (2010)
Thyssen, P.: Identical or distinct? The Paneth–Fajans Debate on the Nature of Isotopes. Manuscript (2023)
Tobin, E.: Crosscutting Natural Kinds and the Hierarchy Thesis. In: Beebee, H., Sabbarton-Leary, N. (eds.) The Semantics and Metaphysics of Natural Kinds, pp. 179–191. Routledge, Abingdon (2010a)
Tobin, E.: Microstructuralism and macromolecules: the case of moonlighting proteins. Found. Chem. 12, 41–54 (2010b). https://doi.org/10.1007/s10698-009-9078-5
Tobin, E.: The metaphysics of determinable kinds. In: Bird, A., Ellis, B., Sankey, H. (eds.) Properties, Powers and Structures. Issues in the Metaphysics of Realism. Routledge, New York, NY, pp. 219–232 (2012)
Tobin, E.: Are natural kinds and natural properties distinct? In: Mumford, S., Tugby, M. (eds.) Metaphysics and Science. Mind Association Occasional Series, Oxford. Chap. 8, pp. 164–182. Oxford University Press (2013)
Van Brakel, J.: Philosophy of Chemistry. Leuven University Press, Leuven (2000)
VanderWerf, C.A.: Acids, Bases, and the Chemistry of the Covalent Bond. D. Van Nostrand Company, New York, NY (1961)
VandeWall, H.: Why water is not H2O, and other critiques of essentialist ontology from the philosophy of chemistry. Philos. Sci. 74, 906–919 (2007)
Walden, P.: Salts, Acids and Bases: Electrolytes: Stereochemistry. McGraw-Hill Book Company, New York (1929)
Williams, N.: Putnam’s traditional neo-essentialism. Philos. Q. 61, 151–170 (2011)
Woodward, J.: Levels, kinds and multiple realizability: the importance of what does not matter. In: Ioannidis, S. et al. (eds.) Levels of Reality in Science and Philosophy. Jerusalem Studies in Philosophy and History of Science, Chap. 14, pp. 261–292. Springer Cham, Switzerland (2022). https://doi.org/10.1007/978-3-030-99425-9_14
Woody, A., Glymour, C.: Missing elements: what philosophers of science might discover in chemistry. In: Bushan, N., Rosenfeld, S. (eds.) Of Minds and Molecules: New Philosophical Perspectives on Chemistry, pp. 17–33. Oxford University Press, New York (2000)
Acknowledgements
I would like to thank Francesca Bellazzi, Hasok Chang, Milenko Lasnibat, Juan Camilo Martínez, Klaus Ruthenberg, Eric Scerri, Jeffrey I. Seeman, and Tuomas Tahko for helpful comments and stimulating discussions, as well as the participants of the following workshops and conferences: the online Centenary Workshop on the Bifurcation of Acidity (Protonism vs. Electronism), organised by Klaus Ruthenberg on March 7, 2023, the one-day workshop on multiple realisability in chemistry, biochemistry and biology, at the Department of Philosophy of the University of Vienna (Austria), organised by the ERC projects Possible Life (PI: Prof. Dr. Tarja Knuuttila, Vienna) and MetaScience (PI: Prof. Dr. Tuomas Tahko, Bristol) on June 5, 2023, the 27th annual summer symposium of the International Society for the Philosophy of Chemistry (ISPC) in Buenos Aires (Argentina) from 18–21 July 2023, and the 17th edition of the International Congress on Logic, Methodology and Philosophy of Science and Technology (CLMPST) in Buenos Aires (Argentina) from 24–29 July 2023.
Funding
I gratefully acknowledge the Fund for Scientific Research (F.R.S.-FNRS) for funding this postdoctoral project (Grant Number 40005996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedicated to the memory of Gilbert N. Lewis, who first proposed his acid–base definitions in 1923, a century ago.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thyssen, P. Are acids natural kinds?. Found Chem (2023). https://doi.org/10.1007/s10698-023-09485-8
Published:
DOI: https://doi.org/10.1007/s10698-023-09485-8
Keywords
- Brønsted–Lowry acids
- Lewis acids
- Primary acid
- Secondary acid
- Natural kinds
- Functional kinds
- Chemical kinds
- Bifurcating kinds
- Essentialism
- Microstructuralism
- Microessentialism
- Multiple realization
- Multiple determination
- Ontological reductionism
- Hierarchy requirement
- Protonism
- Electronism
- Amphoterism
- Dispositional properties
- Powers