Abstract
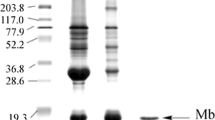
Although myoglobin (Mb) has been considered to be one of the well-characterized proteins, screening of post-genomic era databases revealed the lack of adequate information on teleost Mbs. The present study was aimed to investigate stability and functional features of Mbs from three teleosts of the same family. To unfold how primary structure influences the stability and function of proteins, Mbs were purified from the dark muscles of three carangids, namely, yellowtail, greater amberjack, and silver trevally. Thermostabilities measured by circular dichroism (CD) spectrometry revealed species-specific thermal denaturation pattern, i.e., silver trevally > yellowtail > greater amberjack Mbs. On the other hand, autoxidation rate constants of the ferrous forms of those three carangid Mbs showed positive correlation between the ferrous state of the heme iron and rising temperature. The order of autoxidation rate was in the order of greater amberjack > yellowtail > silver trevally Mbs. The finding of the present study denotes that the thermal stability is not necessarily correlated with the functional stability of carangid Mbs even though their primary structures shared high homology (84–94%).







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeyemi KD, Shittu RM, Sabow AB, Karim R, Sazili AQ (2016) Myofibrillar protein, lipid and myoglobin oxidation, antioxidant profile, phsyicochemical and sensory properties of caprine longissimus thoracis during postmortem conditioning. J Food Process Preserv 41:1–14
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Company, Amsterdam
Aojula HS, Wilson MT, Morrison IG (1987) Functional consequences of haem orientational disorder in sperm-whale and yellow-fin-tuna myoglobins. Biochem J 243:205–210
Bannikov AF (1987) On the taxonomy, composition and origin of the family Carangidae. J Ichthyol 27:1–8
Birnbaum GI, Evans SV, Przybylska M, Rose DR (1994) 1.70 Å resolution structure of myoglobin from yellowfin tuna. An example of a myoglobin lacking the D helix. Acta Crystallogr D Biol Crystallogr 50:283–289
Birukou I, Schweers RL, Olson JS (2010) Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin. J Biol Chem 285:8840–8854
Bismuto E, Gratton E, Lamb DC (2001) Dynamics of ANS binding to tuna apomyoglobin measured with fluorescence correlation spectroscopy. Biophys J 81:3510–3521
Bonaventura C, Henkens R, Alayash AI, Banerjee S, Crumbliss AL (2013) Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxi Redox Signal 18:2298–2313
Brantley REJ, Smerdon SJ, Wilkinson AJ, Singleton EW, Olson JS (1993) The mechanism of autoxidation of myoglobin. J Biol Chem 268:6995–7010
Broumand H, Ball CO, Stier EF (1958) Factors affecting the quality of prepackaged meat. II. E. Determining the proportions of heme derivatives in fresh meat. Food Technol 12:65
Brunori M (2001) Nitric oxide moves myoglobin centre stage. Trends Biochem Sci 26:21–23
Cashon RE, Vayda ME, Sidell BD (1997) Kinetic characterization of myoglobins from vertebrates with vastly different body temperatures. Comp Biochem Physiol – Part B Biochem Mol Biol 117:613–620
Chanthai S, Neida H, Ogawa M, Tamiya T, Tsuchiya T (1996) Studies on thermal denaturation of fish myoglobins using differential scanning calorimetry, circular dichroism, and tryptophan fluorescence. Fish Sci 62:927–932
Chen LC, Lin SB, Chen HH (2004) Thermal stability and denaturation rate of myoglobin from various species of fish. Fish Sci 70:293–298
Chow CJ (1991) Relationship between the stability and autoxidation of myoglobin. J Agric Food Chem 39:22–26
Chow CJ, Ochiai Y, Watabe S, Hashimoto K (1989) Reduced stability and accelerated autoxidation of tuna myoglobin, in association with freezing and thawing. J Agric Food Chem 37:1391–1395
Chow CJ, Wu JC, Lee PF, Ochiai Y (2009) Structural and autooxidation profiles of myoglobins from three species and one hybrid of tilapia (Cichlidae, Perciformes). Comp Biochem Physiol – Part B Biochem Mol Biol 154:274–281
Conforth D (1994) Color-its basis and importance. In: Pearson AM, Duston TR (eds) Quality attributes and their measurement in meat, poultry and fish products. Advances in Meat Research, vol 9. Blackie Academic and Professional, London, pp 34–78
Corrêa DHA, Ramos CHI (2009) The use of circular dichroism spectroscopy to study protein folding, form and function. Afr J Biochem Res 3:164–173
Dasmeh P, Kepp KP, Davis RW (2013) Aerobic dive limits of seals with mutant myoglobin using combined thermochemical and physiological data. Comp Biochem Physiol A 164:119–128
Dong H, Sharma M, Zhou HX, Cross TA (2012) Glycines: role in α-helical membrane protein structures and a potential indicator for native conformation. Biochem 51:4779–4789
Edman P (1950) Method for determination of the amino acid sequence in peptides. Acta Chem Scand 4:283–293
Faustman C, Cassens RG (1990) The biochemical basis for discoloration in fresh meat: a review. J Muscle Foods 1:217–243
Fosmire GJ, Brown WD (1976) Yellowfin tuna (Thunnus albacares) myoglobin: characterization and comparative stability. Comp Biochem Physiol B 55:293–299
Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1:2876–2890
Greer-Walker MG, Pull CA (1975) A survery of red and white muscle in marine fish. J Fish Biol 7:295–300
Gutzke D, Trout GR (2002) Temperature and pH dependence of the autoxidation rate of bovine, ovine, porcine, and cervine oxymyoglobin isolated from three different muscle-longissimus dorsi, gluteus medius, and biceps femoris. J Agric Food Chem 50:2673–2678
Haard NF (1992) Biochemistry and chemistry of color and color change in seafoods. In: Advance in seafood biochemistry. (Flick GJ and Martin RE eds.). Technomic Publishing Co., Inc. USA.
Hamoir G (1953) Myoglobin from carp muscle. Nature 171:345–346
Hasan MM, Watabe S, Ochiai Y (2012) Structural characterization of carangid fish myoglobins. Fish Physiol Biochem 38:1311–1322
Helbo S, Fago A (2011) Allosteric modulation by S-nitrosation in the low-O2 affinity myoglobin from rainbow trout. Am J Physiol Integr Comp Physiol 300:R101–R108
Joseph P, Suman SP, Li S, Fontaine M, Steinke L (2012) Amino acid sequence of myoglobin from white-tailed deer (Odocoileus virginianus). Meat Sci 92:160–163
Kannan G, Kouakou B, Gelaye S (2001) Color changes reflecting myoglobin and lipid oxidation in chevon cuts during refrigerated display. Small Ruminant Res 42:67–75
Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR, Phillips DC, Shore VC (1960) Structure of myoglobin: a three-dimensional Fourier synthesis at 2 Å. resolution. Nature 185:422–427
Kitahara Y, Matsuoka A, Kobayashi N, Shikama K (1990) Autoxidation of myoglobin from bigeye tuna fish (Thunnus obesus). Biochim Biophys Acta 1038:23–28
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Livingston DJ, Brown WD (1981) The chemistry of myoglobin and its reactions. Food Technol 25:256–275
Madden PW, Babcock MJ, Vayda ME, Cashon RE (2004) Structural and kinetic characterization of myoglobins from eurythermal and stenothermal fish species. Comp Biochem Physiol – Part B Biochem Mol Biol 137:341–350
Marcinek DJ, Bonaventura J, Wittenberg JB, Block BA (2001) Oxygen affinity and amino acid sequence of myoglobins from endothermic and ectothermic fish. Am J Physiol Regul Integr Comp Physiol 208:R1123–R1133
Matsuura F, Hashimoto K (1954) Determination of the content of hemoglobin, myoglobin and cytochrome C in the muscles of fishes. Bull Japan Soc Sci Fish 20:308–312
Matsuura F, Hashimoto K (1959) Chemical studies on the red muscle (chiai) of fishes. Bull Japan Soc Sci Fish 24:809–815
Mirceta S, Signore AV, Burns JM, Cossins AR, Campbell KL, Berenbrink M (2013) Evolution of mammalian diving capacity traced by myoglobin net surface charge. Science 340:1234192
Naylor GJP, Gerstein M (2000) Measuring shifts in function and evolutionary opportunity using variability profiles: a case study of the globins. J Mol Evol 51:223–233
Nicola NA, Minasian E, Appleby CA, Leach SJ (1975) Circular dichroism studies of myoglobin and leghemoglobin. Biochemistry 14:5141–5149
Nurilmala M, Ushio H, Ochiai Y (2018) pH- and temperature- dependent denaturation profiles of tuna myoglobin. Fish Sci 84:579–587
Ochiai Y, Ueki N, Watabe S (2009) Effects of point mutations on the structural stability of tuna myoglobins. Comp Biochem Physiol – Part B Biochem Mol Biol 153:223–228
Ochiai Y, Watanabe Y, Ozawa H, Ikegami S, Uchida N, Watabe S (2010) Thermal denaturation profiles of tuna myoglobin. Biosci Biotechnol Biochem 74:1673–1679
Ordway GA, Garry DJ (2004) Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol 207:3441–3446
Ramos CHI, Ferreira ST (2005) Protein folding, misfolding and aggregation: evolving concepts and conformational diseases. Protein Pept Lett 12:213–222
Rayner BS, Wu BJ, Raftery M, Stocker R, Witting PK (2005) Human S-nitroso oxymyoglobin is a store of vasoactive nitric oxide. J. Biol. Chem. 280:9985–9993
Regis WCB, Fattori J, Santoro MM, Jamin M, Ramos CHI (2005) On the difference in stability between horse and sperm whale myoglobins. Arch Biochem Biophys 436:168–177
Rossi-Fanelli A, Antonini E (1955) Purification and crystallization of the myoglobin of salt water fish. Arch Biochem Biophys 58:498–500
Saud S, Li G, Sun Y, Khan MI, Rehman AU, Uzzaman A, Liu W, Ding C, Xiao H, Wang Y, Cao C (2019) A facile isoelectric focusing of myoglobin and hemoglobin used as markers for screening of chicken meat quality in China. Electrophoresis 20:2767–2774
Schreiter ER, Rodriguez MM, Weichsel A, Montfort WR, Bonaventura J (2007) S-nitrosylation-induced conformational change in blackfin tuna myoglobin. J Biol Chem 282:19773–19780
Schulz GE, Schirmer RH (1979) Principles of protein structure. Springer-Verlag, New York, USA
Shikama K, Matsuoka A (1994) Aplysia myoglobin with unusual properties: another prototype in myoglobin and haemeglobin biochemistry. Biol Rev 69:233–251
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Springer BA, Egeberg KD, Sligar SG, Rohlfs RJ, Mathews AJ, Olson JS (1989) Discrimination between oxygen and carbon monoxide and inhibition of autoxidation by myoglobin. Site-directed mutagenesis of the distal histidine. J Biol Chem 264:3057–3060
Strehlow KG, Baldwin RL (1989) Effect of the substitution Ala→ Gly at each of five residue positions in the C-peptide helix. Biochemistry 28:2130–2133
Suzuki N, Hashimoto K, Matsuura F (1973) Studies on the colour of skipjack meat. Bull Japan Soc Sci Fish 39:35–41
Tada T, Watanabe Y, Matsuoka A, Ikeda-Saito M, Imai K, Ni-Hei Y, Shikama K (1998) African elephant myoglobin with an unusual autoxidation behavior: comparison with the H64Q mutant of sperm whale myoglobin. Biochem Biophys Acta 1387:165–176
Ueki N, Ochiai Y (2005) Structural stabilities of recombinant scombridae fish myoglobins. Biosci Biotechnol Biochem 69:1935–1943
Ueki N, Ochiai Y (2006) Effect of amino acid replacements on the structural stability of fish myoglobin. J Biochem 140:649–656
Ueki N, Chow CJ, Ochiai Y (2005) Characterization of bullet tuna myoglobin with reference to the thermostability-structure relationship. J Agric Food Chem 53:4968–4975
Whitaker TL, Berry MB, Ho EL, Hargrove MS, Philips GN, Komiyama NH, Nagai K, Olson JS (1995) The D-Helix in myoglobin and in the β subunit of hemoglobin is required for the reduction of heme. Biochemistry 34:8221–8226
Wiechelman KJ, Braun RD, Fitzpatrick JD (1988) Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem 175:231–237
Wittenberg JB, Wittenberg BA (2003) Myoglobin function reassessed. J Exp Biol 206:2011–2020
Woody RW (1995) Circular dichroism. Method Enzymol 246:34–71
Yamada T, Takakura H, Jue T, Hashimoto T, Ishizawa R, Furuichi Y, Kato Y, Iwanaka N, Masuda K (2016) Myoglobin and the regulation of mitochondrial respiratory chain complex IV. J Physiol 594:483–495
Funding
This work was partly supported by the Japan Society for Promotion of Sciences (KAKENHI # 22380015 to Y. O.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Muhammad Mehedi Hasan, Hideo Ozawa, and Purnama Arafah. The first draft of the manuscript was written by Muhammad Mehedi Hasan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Consent to participate is not applicable
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.19 mb)
Rights and permissions
About this article
Cite this article
Hasan, M.M., Arafah, P., Ozawa, H. et al. Thermal denaturation and autoxidation profiles of carangid fish myoglobins. Fish Physiol Biochem 47, 487–498 (2021). https://doi.org/10.1007/s10695-021-00928-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00928-7