Abstract
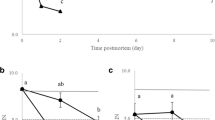
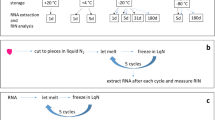
Studies of post-mortem interval on the stability of RNA from a number of various mammals have shown RNA to be stable for between 24 and 48 h following death. As yet there have been no studies looking at RNA stability in post-mortem tissues of poikilothermic fish. Brain, kidney, liver and muscle were collected from Atlantic salmon (Salmo salar) parr and samples of each tissue were placed into RNAlater™ after 0–24 h post-mortem storage at room temperature. Electrophoretic analysis of the total RNA showed degradation of ribosomal RNA only in muscle from 8 h onwards. Probing of northern blots with β-actin showed that, in the brain, β-actin mRNA was stable for 24 h post-mortem but degradation of mRNA was observed after 8 h with the kidney and liver and after 4 h with the muscle. Expression of the weakly expressed thyroid hormone receptor β (TRβ) was detected by reverse transcriptase polymerase chain reaction (RT-PCR) in all tissues up to 24 h post-mortem although a reduction in PCR product was observed after 8 h with muscle and 24 h with kidney. Analysis with an Agilent 2100 Bioanalyzer showed that the RNA integrity number (RIN) of brain total RNA remained constant for 8 h post-mortem with only a small fall at 24 h post-mortem. The RINs of the remaining tissues indicated degradation at 8 h post-mortem with kidney and muscle and at 24 hours post-mortem with liver. Taken together these findings show that degradation of Atlantic salmon RNA is tissue dependent but stable for at least one hour post-mortem.



Similar content being viewed by others
Abbreviations
- RIN:
-
RNA integrity number
- TRβ:
-
Thyroid hormone receptor β
References
Catts VS, Catts SV, Fernandez HR, Taylor JM, Coulson EJ, Lutze-Mann LH (2005) A microarray study of post-mortem mRNA degradation in mouse brain tissue. Mol Brain Res 138:164–177
Fitzpatrick R, Casey OM, Morris D, Smith T, Powell R, Sreenan JM (2002) Post-mortem stability of RNA isolated from bovine reproductive tissues. Biochim Biophys Acta 1574:10–14
Fleige S, Pfaffl MW (2006) RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med 27:126–129
Imbeaud S, Graudens E, Boulanger V, Barlet X, Zaborski P, Eveno E, Mueller O, Schroeder A, Auffray C (2005) Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res 33:e56
Inoue H, Kimara A, Tuji T (2002) Degradation profile of mRNA in a dead rat body: basic semi-quantification study. Forensic Sci Int 130:127–132
Johnson SA, Morgan DG, Finch CE (1986) Extensive post-mortem stability of RNA from rat and human brain. J Neurosci Res 16:267–280
Malik KJ, Chen CD, Olsen TW (2003) Stability of RNA from the retina and retinal pigment epithelium in a porcine model simulating human eye bank conditions. Invest Opthalmol Vis Sci 44:2730–2735
Marchuk L, Sciore P, Reno C, Frank CB, Hart DA (1998) Post-mortem stability of total RNA isolated from rabbit ligament, tendon and cartilage. Biochim Biophys Acta 1379:171–177
Preece P, Cairns NJ (2003) Quantifying mRNA in post-mortem brains: influence of gender, age at death, post-mortem interval, brain pH, agonal state and inter-lobe mRNA variance. Mol Brain Res 118:60–71
Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3
Tourrière H, Chebli K, Tazi J (2002) mRNA degradation machines in eukaryotic cells. Biochimie 84:821–837
Trotter SA, Brill II LB, Bennett JP Jr (2002) Stability of gene expression in post-mortem brain revealed by cDNA gene array analysis. Brain Res 942:120–123
Wetzel DM, Bohn MC, Hamill RW (1994) Post-mortem stability of mRNA for glucocorticoid and mineralocorticoid receptor in rodent brain. Brain Res 649:117–121
Wilusz CJ, Wormington M, Peltz SW (2001) The cap-to-tail guide to mRNA turnover. Nature Rev Mol Cell Biol 2:237–246
Acknowledgements
The authors would like to thank Dr Sarah Rogers for supplying the Atlantic salmon β-actin clone and Dr Marco Campinho for his technical assistance. This work was supported by the Biotechnology and Biological Sciences Research Council under grant no. 72/EGA17676.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seear, P.J., Sweeney, G.E. Stability of RNA isolated from post-mortem tissues of Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 34, 19–24 (2008). https://doi.org/10.1007/s10695-007-9141-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-007-9141-x