Abstract
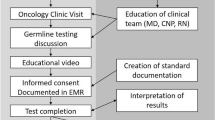
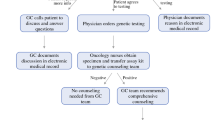
Hereditary predisposition is estimated to account for 10% of all pancreatic cancer cases. However, referral patterns and clinical workflow for germline testing in this disease differ significantly by institution, and many at-risk patients may not undergo appropriate counseling and testing. We undertook an analysis of patients diagnosed with pancreatic cancer (PDAC) who were referred to the Clinical Genetics program of a high-volume academic center over a 3-year period to assess referral frequency, evaluate the yield of germline testing in this selected patient cohort, and elucidate the reasons individuals did not undergo recommended germline testing. Medical records of patients with PDAC referred for genetic counseling between January 2015 and October 2017 were reviewed for demographic, medical/family history, and disease-specific data. If testing did not occur, reasons were documented. Genetic test results were categorized as negative, variants of unknown significance, or established pathogenic mutations. Descriptive statistics included means with standard deviations; associations were analyzed with t test and Fisher’s exact test. 32% (137 of 432) of PDAC patients were referred for genetic counseling, but only 64% attended their appointment and 60% ultimately underwent germline testing. Common reasons for attrition included worsening disease severity, lack of patient follow-up, insurance concerns, and logistic/travel challenges. Pathogenic germline mutations were detected in 20% (16 of 82) of patients tested, distributed across races/ethnicities, and significantly associated with younger age and positive family history of breast cancer. PDAC patients frequently do not undergo genetic counseling/germline testing despite appropriate referrals, highlighting a need to develop streamlined processes to engage more patients in testing, especially those with high-risk features.

Similar content being viewed by others
References
Peters ML, Tseng JF, Miksad RA (2016) Genetic testing in pancreatic ductal adenocarcinoma: implications for prevention and treatment. Clin Ther 38(7):1622–1635. https://doi.org/10.1016/j.clinthera.2016.03.006
Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, Goggins M (2017) Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 35(30):3382–3390. https://doi.org/10.1200/jco.2017.72.3502
Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J, Mandelker D, Zehir A, Capanu M, Salo-Mullen E, Arnold AG, Yu KH, Varghese AM, Kelsen DP, Brenner R, Kaufmann E, Ravichandran V, Mukherjee S, Berger MF, Hyman DM, Klimstra DS, Abou-Alfa GK, Tjan C, Covington C, Maynard H, Allen PJ, Askan G, Leach SD, Iacobuzio-Donahue CA, Robson ME, Offit K, Stadler ZK, O’Reilly EM (2018) Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djy024
NCCN Clinical Practice Guidelines in Oncology: Pancreatic cancer, version 2.2018 https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 16 Sept 2018
NCCN Clinical Practice Guidelines in Oncology: Genetic/familial high-risk assessment (breast and ovarian), version 2.2019 https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed 16 Sept 2018
Groman JD, Hefferon TW, Casals T, Bassas L, Estivill X, Des Georges M, Guittard C, Koudova M, Fallin MD, Nemeth K, Fekete G, Kadasi L, Friedman K, Schwarz M, Bombieri C, Pignatti PF, Kanavakis E, Tzetis M, Schwartz M, Novelli G, D’Apice MR, Sobczynska-Tomaszewska A, Bal J, Stuhrmann M, Macek M Jr, Claustres M, Cutting GR (2004) Variation in a repeat sequence determines whether a common variant of the cystic fibrosis transmembrane conductance regulator gene is pathogenic or benign. Am J Hum Genet 74(1):176–179. https://doi.org/10.1086/381001
Malats N, Casals T, Porta M, Guarner L, Estivill X, Real FX (2001) Cystic fibrosis transmembrane regulator (CFTR) DeltaF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. PANKRAS II study group. Gut 48(1):70–74
Weiss FU, Simon P, Bogdanova N, Mayerle J, Dworniczak B, Horst J, Lerch MM (2005) Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut 54(10):1456–1460. https://doi.org/10.1136/gut.2005.064808
Petersen GM (2016) Familial pancreatic cancer. Semin Oncol 43(5):548–553. https://doi.org/10.1053/j.seminoncol.2016.09.002
Hruban RH, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Falatko F, Yeo CJ, Kern SE (1999) Familial pancreatic cancer. Ann Oncol 10(Suppl 4):69–73
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116(6):1453–1456
Cybulski KE, Howlett NG (2011) FANCP/SLX4: a Swiss army knife of DNA interstrand crosslink repair. Cell Cycle 10(11):1757–1763. https://doi.org/10.4161/cc.10.11.15818
Li D, Suzuki H, Liu B, Morris J, Liu J, Okazaki T, Li Y, Chang P, Abbruzzese JL (2009) DNA repair gene polymorphisms and risk of pancreatic cancer. Clin Cancer Res 15(2):740–746. https://doi.org/10.1158/1078-0432.Ccr-08-1607
Bahra M, Kamphues C, Boas-Knoop S, Lippert S, Esendik U, Schuller U, Hartmann W, Waha A, Neuhaus P, Heppner F, Pietsch T, Koch A (2012) Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas 41(2):222–229. https://doi.org/10.1097/MPA.0b013e31822896dd
Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S (2014) Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111(6):1132–1138. https://doi.org/10.1038/bjc.2014.418
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361(2):123–134. https://doi.org/10.1056/NEJMoa0900212
Lee JM, Ledermann JA, Kohn EC (2014) PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol 25(1):32–40. https://doi.org/10.1093/annonc/mdt384
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409–413. https://doi.org/10.1126/science.aan6733
Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, Lowery MA, Diaz LA Jr, Mandelker D, Yu KH, Zervoudakis A, Kelsen DP, Iacobuzio-Donahue CA, Klimstra DS, Saltz LB, Sahin IH, O’Reilly EM (2018) Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res 24(6):1326–1336. https://doi.org/10.1158/1078-0432.Ccr-17-3099
Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S, Chang DK, Miller DK, Pajic M, Kassahn KS, Quinn MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Stone A, Wilson PJ, Anderson M, Fink JL, Holmes O, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Mead RS, Xu Q, Wu J, Pinese M, Cowley MJ, Jones MD, Nagrial AM, Chin VT, Chantrill LA, Mawson A, Chou A, Scarlett CJ, Pinho AV, Rooman I, Giry-Laterriere M, Samra JS, Kench JG, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, McKay CJ, Carter CR, Dickson EJ, Graham JS, Duthie F, Oien K, Hair J, Morton JP, Sansom OJ, Grutzmann R, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Rusev B, Corbo V, Salvia R, Cataldo I, Tortora G, Tempero MA, Hofmann O, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Gill AJ, Pearson JV, Grimmond SM, Waddell N, Biankin AV (2017) Hypermutation In pancreatic cancer. Gastroenterology 152(1):68–74.e62. https://doi.org/10.1053/j.gastro.2016.09.060
Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL (2015) A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17(1):70–87. https://doi.org/10.1038/gim.2014.147
Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, Dhani N, Narod S, Akbari M, Moore M, Gallinger S (2015) Germline BRCA Mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33(28):3124–3129. https://doi.org/10.1200/jco.2014.59.7401
Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, Bamlet WR, Chaffee KG, DiCarlo J, Wu Z, Samara R, Kasi PM, McWilliams RR, Petersen GM, Couch FJ (2018) Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 319(23):2401–2409. https://doi.org/10.1001/jama.2018.6228
Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, Shields AE (2011) Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med 13(4):349–355. https://doi.org/10.1097/GIM.0b013e3182091ba4
Forman AD, Hall MJ (2009) Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J 15(Suppl 1):S56–S62. https://doi.org/10.1111/j.1524-4741.2009.00798.x
Matro JM, Ruth KJ, Wong YN, McCully KC, Rybak CM, Meropol NJ, Hall MJ (2014) Cost sharing and hereditary cancer risk: predictors of willingness-to-pay for genetic testing. J Genet Couns 23(6):1002–1011. https://doi.org/10.1007/s10897-014-9724-5
Wideroff L, Vadaparampil ST, Breen N, Croyle RT, Freedman AN (2003) Awareness of genetic testing for increased cancer risk in the year 2000 National Health Interview Survey. Community Genet 6(3):147–156. https://doi.org/10.1159/000078162
Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, Lipkin SM, Syngal S, Wollins DS, Lindor NM (2015) American Society of Clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 33(31):3660–3667. https://doi.org/10.1200/jco.2015.63.0996
Beitsch PD, Whitworth PW (2014) Can breast surgeons provide breast cancer genetic testing? An American Society of breast surgeons survey. Ann Surg Oncol 21(13):4104–4108. https://doi.org/10.1245/s10434-014-3711-9
Cragun D, Camperlengo L, Robinson E, Caldwell M, Kim J, Phelan C, Monteiro AN, Vadaparampil ST, Sellers TA, Pal T (2015) Differences in BRCA counseling and testing practices based on ordering provider type. Genet Med 17(1):51–57. https://doi.org/10.1038/gim.2014.75
Vadaparampil ST, Scherr CL, Cragun D, Malo TL, Pal T (2015) Pre-test genetic counseling services for hereditary breast and ovarian cancer delivered by non-genetics professionals in the state of Florida. Clin Genet 87(5):473–477. https://doi.org/10.1111/cge.12405
Keiles S, Kammesheidt A (2006) Identification of CFTR, PRSS1, and SPINK1 mutations in 381 patients with pancreatitis. Pancreas 33(3):221–227. https://doi.org/10.1097/01.mpa.0000232014.94974.75
Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA (2001) Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology 121(6):1310–1319
Grody WW, Cutting GR, Klinger KW, Richards CS, Watson MS, Desnick RJ (2001) Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genet Med 3(2):149–154. https://doi.org/10.1097/00125817-200103000-00010
Han FF, Guo CL, Liu LH (2013) The effect of CHEK2 variant I157T on cancer susceptibility: evidence from a meta-analysis. DNA Cell Biol 32(6):329–335. https://doi.org/10.1089/dna.2013.1970
Kleibl Z, Havranek O, Hlavata I, Novotny J, Sevcik J, Pohlreich P, Soucek P (2009) The CHEK2 gene I157T mutation and other alterations in its proximity increase the risk of sporadic colorectal cancer in the Czech population. Eur J Cancer 45(4):618–624. https://doi.org/10.1016/j.ejca.2008.09.022
Win AK, Cleary SP, Dowty JG, Baron JA, Young JP, Buchanan DD, Southey MC, Burnett T, Parfrey PS, Green RC, Le Marchand L, Newcomb PA, Haile RW, Lindor NM, Hopper JL, Gallinger S, Jenkins MA (2011) Cancer risks for monoallelic MUTYH mutation carriers with a family history of colorectal cancer. Int J Cancer 129(9):2256–2262. https://doi.org/10.1002/ijc.25870
Rennert G, Lejbkowicz F, Cohen I, Pinchev M, Rennert HS, Barnett-Griness O (2012) MutYH mutation carriers have increased breast cancer risk. Cancer 118(8):1989–1993. https://doi.org/10.1002/cncr.26506
Kentwell M, Dow E, Antill Y, Wrede CD, McNally O, Higgs E, Hamilton A, Ananda S, Lindeman GJ, Scott CL (2017) Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol 145(1):130–136. https://doi.org/10.1016/j.ygyno.2017.01.030
Percival N, George A, Gyertson J, Hamill M, Fernandes A, Davies E, Rahman N, Banerjee S (2016) The integration of BRCA testing into oncology clinics. Br J Nurs 25(12):690–694. https://doi.org/10.12968/bjon.2016.25.12.690
George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, Cloke V, Slade I, Kemp Z, Gore M, Strydom A, Banerjee S, Hanson H, Rahman N (2016) Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep 6:29506. https://doi.org/10.1038/srep29506
Acknowledgements
Dr. Andrew H. Ko has received research funding (paid directly to his institution, not to him personally) to help support the conduct of clinical trials in pancreatic cancer from Merrimack, Halozyme, Roche/Genentech, and Celgene. During the past year he has received compensation for consulting or serving on advisory or data safety monitoring boards specific to pancreatic cancer from Pharmacyclics, Celgene, ARMO Biosciences, and Astra Zeneca. None of these commercial entities provided funding for this work, nor were involved in the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors report no conflicts of interest relevant to the information presented in this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Walker, E.J., Carnevale, J., Pedley, C. et al. Referral frequency, attrition rate, and outcomes of germline testing in patients with pancreatic adenocarcinoma. Familial Cancer 18, 241–251 (2019). https://doi.org/10.1007/s10689-018-0106-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-018-0106-2