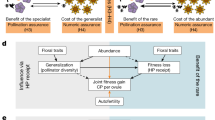
Abstract
It has been proposed frequently, from Darwin’s time onwards, that specialized pollination increases speciation rates and thus the diversity of plant species (i.e. clade species richness). We suggest here that the correlation between clade species richness and floral specialization is real, but that clade species richness is frequently the cause, not the result of floral specialization. We urge a broader, variance-partitioning perspective for assessing the causes of this correlation by suggesting four models of how the diversity-specialization correlation might come about: (1) floral specialization promotes initial reproductive isolation (“Initial-RI” model), (2) floral specialization promotes reinforcement of reproductive isolation upon secondary contact (“Reinforcement” model), (3) floral specialization reduces the extinction rate by promoting tighter species packing (“Extinction” model), (4) floral specialization is the result of high clade species richness, which increases the number of related species in communities, and thus selects for floral character displacement (“Character-Displacement” model). These hypotheses are evaluated by comparing the relationships between species richness, speciation mechanisms, and pollination precision, accuracy, and specialization in the broader literature and, more specifically, in four study systems: Dalechampia (Euphorbiaceae), Collinsia (Plantaginaceae), Burmeistera (Campanulaceae), and Stylidium (Stylidiaceae). These systems provide stronger support for the character-displacement hypothesis, wherein local species diversity drives the evolution of specialized pollination. Although the two reproductive-isolation hypotheses may hold for plants like orchids, with extremely precise pollination systems, the reproductive character-displacement hypothesis seems likely to be more important for plant groups with less precise pollination systems.


Similar content being viewed by others
References
Aldridge G, Campbell DR (2007) Variation in pollinator preference between two Ipomopsis contact sites that differ in hybridization rate. Evolution 61:99–110
Armbruster WS (1985) Patterns of character divergence and the evolution of reproductive ecotypes of Dalechampia scandens (Euphorbiaceae). Evolution 39:733–752
Armbruster WS (1986) Reproductive interactions between sympatric Dalechampia species: are natural assemblages "random" or organized? Ecology 67:522–533
Armbruster WS (1988) Multilevel comparative analysis of morphology, function, and evolution of Dalechampia blossoms. Ecology 69:1746–1761
Armbruster WS (1990) Estimating and testing the shapes of adaptive surfaces: the morphology and pollination of Dalechampia blossoms. Am Nat 135:14–31
Armbruster WS (1993) Evolution of plant pollination systems: hypotheses and tests with the neotropical vine Dalechampia. Evolution 47:1480–1505
Armbruster WS (1997) Exaptations link the evolution of plant-herbivore and plant-pollinator interactions: a phylogenetic inquiry. Ecology 78:1661–1674
Armbruster WS (2006) Evolutionary and ecological perspectives on specialization: from the arctic to the tropics. In: Waser N, Ollerton J (eds) Plant-pollinator interactions: from specialization to generalization. University of Chicago Press, Chicago, pp 260–282
Armbruster WS, Herzig AL (1984) Partitioning and sharing of pollinators by four sympatric species of Dalechampia (Euphorbiaceae) in Panama. Ann Missouri Botanical Garden 71:1–16
Armbruster WS, Steiner KE (1992) Pollination ecology of four Dalechampia species (Euphorbiaceae) in northern Natal, South Africa. Am J Bot 79:306–313
Armbruster WS, Webster GL (1982) Divergent pollination systems in sympatric species of South American Dalechampia (Euphorbiaceae). Am Midl Nat 108:325–337
Armbruster WS, Herzig AL, Clausen TP (1992) Pollination of two sympatric species of Dalechampia (Euphorbiaceae) in Suriname by male euglossine bees. Amer J Bot 79:1374–1381
Armbruster WS, Edwards ME, Debevec EM (1994) Character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75:315–329
Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Velasquez Runk JL (1999) Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a reevaluation of Berg’s correlation-pleiades concept. Am J Bot 86:39–55
Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H (2002) Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae, s.l.). Amer J Bot 89:37–49
Armbruster WS, Pélabon C, Hansen TF, Mulder CPH (2004) Floral integration and modularity: distinguishing complex adaptations from genetic constraints. In: Pigliucci M, Preston KA (eds) The evolutionary biology of complex phenotypes, Oxford University Press, Oxford, pp 23–49
Berg RL (1960) The ecological significance of correlation pleiades. Evolution 14:171–180
Bolmgren K, Eriksson O, Linder HP (2003) Contrasting flowering phenology and species richness in abiotically and biotically pollinated angiosperms. Evolution 57:2001–2011
Bradshaw HD, Schemske DW (2003) Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426:176–178
Brown JH, Kodric-Brown A (1979) Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology 60:1022–1035
Butlin R (1989) Reinforcement of premating isolation. In: Otte D, Endler JA (eds) Speciation and its consequences. Sinauer, Sunderland, MA, pp 158–179
Chase MW, Hills HG (1992) Orchid phylogeny, flower sexuality, and fragrance-seeking bees. BioScience 42:43–49
Coates DJ, Carstairs S, Hamley VL (2003) Evolutionary patterns and genetic structure in localized and widespread species in the Stylidium caricifolium complex (Stylidiaceae). Am J Bot 90:997–1008
Cooley AM, Carvallo G, Willis JH (2008) Is floral diversification associated with ollinator divergence? Flower shape, flower colour and pollinator preferencein Chilean Mimulus. Ann Bot 101:641–650
Cozzolino S, Widmer A (2005) Orchid diversity: an evolutionary consequence of deception? Trend Ecol Evol 20:487–494
Cozzolino S, Scopece G (2008) Deceptive orchids: the promise of sex or food and its consequences for reproductive isolation. Phil Trans R Soc B (in press)
Cozzolino S, Schiestl FP, Muller A, De Castro O, Nardella AM, Widmer A (2005) Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: absence of premating barriers? Proc R Soc B Biol Sci 272:1271–1278
Coyne JA, Orr HA (1989) Patterns of speciation in Drosophila. Evolution 43:362–381
Crepet WL (1984) Advanced (constant) insect pollination mechanisms—pattern of evolution and implications vis-a-vis angiosperm diversity. Ann Miss Bot Gard 71:607–630
Cronquist A (1982) An integrated system of classification of flowering plants. Columbia University Press, New York
Darwin C (1876) The effects of cross—and self-fertilization in the vegetable kingdom. John Murray, London
Darwin C (1877) The various contrivances by which orchids are fertilised by insects. Republished 1984, University of Chicago Press, Chicago
Davies TJ, Barraclough TG, Chase MW, Soltis PS, Soltis DE, Savolainen V (2004) Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc Nat Acad Sci USA 101:1904–1909
Diggle PK (1992) Development and the evolution of plant reproductive characters. In: Wyatt R (ed) Ecology and evolution of plant reproduction. Chapman & Hall, New York, pp 326–355
Dobzhansky T (1937) Genetics and the origin of species. Columbia Univ Press, New York
Dodd ME, Silvertown J, Chase MW (2000) Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution 53:732–744
Dodson CH (1962) The importance of pollinators in the evolution of the orchids of tropical America. Am Orchid Soc Bull 31:525–534, 641–649, 731–735
Dressler RL (1968) Pollination by euglossine bees. Evolution 22:202–210
Dressler RL (1981) The orchids: natural history and classification. Harvard University Press, Cambridge, Mass, USA
Ennos RA (2008) Spurred by pollinators. Heredity 100:3–4
Fægri K, van der Pijl L (1979) The principles of pollination ecology, 3rd edn. Pergamon, Oxford, 244 pp
Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JT (2004) Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst 35:375–403
Fishman L, Wyatt R (1999) Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 53:1723–1733
Gegear RJ, Burns JG (2007) The birds, the bees, and the virtual flowers: can pollinator behavior drive ecological speciation in flowering plants? American Naturalist 170:551–566
Goldblatt P, Manning JC, Bernhardt P (1995) Pollination biology of Lapeirousia subgenus Lapeirousia (Iridaceae) in Southern Africa––floral divergence and adaptation for long-tongued fly pollination. Ann Mis Bot Gard 82:517–534
Grant V (1949) Pollination systems as isolating mechanisms in plants. Evolution 3:82–97
Grant V (1966) The selective origin of incompatibility barriers in the plant genus Gilia. Am Nat 100:99–118
Grant V (1971) Plant speciation, 2nd edn. Columbia University Press, New York, NY, USA
Grant V (1994) Modes and origins of mechanical and ethological isolation in angiosperms. Proc Nat Acad Sci USA 91:3–10
Grant V, Grant KA (1965) Pollination in the phlox family. Columbia University Press, New York
Hansen TF, Carter AJR, Pélabon C (2006) On adaptive accuracy and precision in natural populations. Am Nat 168:168–181
Heinrich B. Raven PH (1972) Energetics and pollination ecology. Science 176:597–602
Hersch EI, Roy BA (2007) Context-dependent pollinator behavior: an explanation for patterns of hybridization among three species of Indian paintbrush. Evolution 61:111–124
Hodges SA (1997) Floral nectar spurs and diversification. Int J Plant Sci 158:S81–S88
Hodges SA, Arnold ML (1995) Spurring plant diversification: are floral nectar spurs a key innovation? Proc R Soc Lond B Biol Sci 262:343–348
Hodges SA, Whittall JB (2008) One-sided evolution or two? A reply to ennos. Heredity (advance online publication) doi:10.1038/hdy.2008.12
Hopper SD (1979) Biogeographical aspects of speciation in the southwest Australian flora. Annu Rev Ecol Syst 10:399–422
Hoskin J, Higgie M, McDonald KR, Moritz C (2005) Reinforcement drives rapid allopatric speciation. Nature 437:1353–1356
Howell DJ (1977) Time sharing and body partitioning in bat-plant pollination systems. Nature 270:509–510
Inoue K, Amano M (1986) Evolution of Campanula punctata Lam. in the Izu Islands: changes of pollinators and evolution of breeding systems. Plant Spec Biol 1:89–97
Jesson LK (2007) Ecological correlates of diversification in New Zealand angiosperm lineages. N Z J Bot 45:35–51
Johnson SD (2007) Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH (eds) Ecology and evolution of flowers. Oxford University Press, Oxford, UK, pp 295–310
Johnson SD, Steiner KE (1997) Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51:45–53
Keller CS, Armbruster WS (1989) Pollination of Hyptis capitata in Panama by eumenid wasps. Biotropica 21:190–192
Knox EB, Muasya AM, Muchhala N (2008) The predominantly South American clade of Lobeliaceae. Sys Bot (in press)
Kozak KH, Larson AA, Bonett RM, Harmon LJ (2005) Phylogenetic analysis of ecomorphological divergence, community structure, and diversification rates in dusky salamanders (Plethodontidae: Desmognathus). Evolution 9:2000–2016
Lukhtanov VA, Kandul NP, Plotkin JB, Dantchenko AV, Haig D, Pierce NE (2005) Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature 436:385–389
Macior LW (1975) The pollination ecology of Pedicularis (Scrophulariaceae) in the Yukon Territory. Am J Bot 62:1065–1072
Macior LW (1983) The pollination dynamics of sympatric species of Pedicularis (Scrophulariaceae). Am J Bot 70:844–853
McKinnon JS, Mori S, Blackman BK, David L, Kingsley DM, Jamieson L, Chou J, Schluter D (2004) Evidence for ecology’s role in speciation. Nature 429:294–298
Miller RB (1981) Hawkmoths and the geographic patterns of floral variation in Aquilegia caerulea. Evolution 35:763–774
Miyake T, Inoue K (2003) Character displacement in style length between pollinator-sharing Clerodendrum trichotomum and C. izuinsulare (Verbenaceae). Plant Syst Evol 243:31–38
Moyle LC, Olson MS, Tiffin P (2004) Patterns of reproductive isolation in three angiosperm genera. Evolution 58:1195–1208
Muchhala N (2003) Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora (Campanulaceae). Oecologia 134:373–380
Muchhala N (2006a) The pollination biology of Burmeistera (Campanulaceae): specialization and syndromes. Am J Bot 93:1081–1089
Muchhala N (2006b) Nectar bat stows huge tongue in its rib cage. Nature 444:701–702
Muchhala N (2007) Adaptive tradeoff in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. Am Nat 169:494–504
Muchhala N (2008) Functional significance of interspecific variation in Burmeistera flower morphology: evidence from bat captures. Biotropica doi:10.1111/j.1744-7429.2007.00381.x
Muchhala N, Potts MD (2007) Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process, and pattern. Proc R Soc B 274:2731–2737
Murcia C, Feinsinger P (1996) Interspecific pollen loss by hummingbirds visiting flower mixtures: effects of floral architecture. Ecology 77:550–560
Nilsson LA (1988) The evolution of flowers with deep corolla tubes. Nature 334:147–149
Nilsson LA, Jonsson L, Ralison L, Randrianjohany E (1987) Angrecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. Biotropica 19:310–318
Noor MAF. 1999. Reinforcement and other consequences of sympatry. Heredity 83:503–508
Ollerton J (1996) Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant-pollinator systems. J Ecol 84:767–769
Ollerton J, Killick A, Lamborn E, Watts S, Whiston M (2007) Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56:717–728
Orr HA (2005) The genetic basis of reproductive isolation: insights from Drosophila. Proc Nat Acad Sci USA 102:6522–6526
Pauw A (2006) Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi, Melittidae) in a guild of South African orchids (Coryciinae). Am J Bot 93:917–926
Pélabon C, Carlson ML, Hansen TF, Armbruster WS (2004) Variational and genetic properties of developmental stability in Dalechampia scandens. Evolution 58:504–514
Pélabon C, Carlson ML, Hansen TF, Armbruster WS (2005) Effects of crossing distance on offspring fitness and developmental stability in Dalechampia scandens (Euphorbiaceae). Am J Bot 92:842–851
Pellmyr O (1986) Three pollination morphs in Cimicifuga simplex—Incipient speciation due to inferiority in competition. Oecologia 68:304–307
Perret M, Chautems A, Spichiger R, Barraclough TG, Savolainen V (2007) The geographical pattern of speciation and floral diversification in the neotropics: the tribe Sinningieae (Gesneriaceae) as a case study. Evolution 61:1641–1660
Raven PH (1977) A suggestion concerning the cretaceous rise to dominance of the angiosperms. Evolution 31(2):451–452
Regal PJ (1977) Ecology and evolution of flowering plant dominance. Science 196:622–629
Rice WR, Hostert EE (1993) Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47:1637–1653
Ricklefs RE, Renner SS (1994) Species richness within families of flowering plants. Evolution 48:1619–1636
Rieseberg LH, Willis JH (2007) Plant speciation. Science 317:910–914
Rieseberg LH, Church SA, Morjan CL (2004) Integration of populations and differentiation of species. New Phytol 161:59–69
Robertson JL, Wyatt R (1990) Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris. Evolution 44:121–33
Roughgarden J (1979) Theory of population genetics and evolutionary ecology: an introduction. MacMillan, New York, 612 pp
Sargent RD (2004) Floral symmetry affects speciation rates in angiosperms. Proc R Soc Lond B Biol Sci 271:603–608
Schemske DW, Bradshaw HD (1999) Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc Nat Acad Sci USA 96:11910–11915
Scopece G, Musacchio A, Widmer A, Cozzolino S (2007) Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution 61:2623–2642
Servedio MR, Noor MAF (2003) The role of reinforcement in speciation: theory and data. Ann Rev Ecol Syst 34:339–364
Smith SA, Rausher MD (2007) Experimental evidence that selection favors character displacement in the ivyleaf morning glory. Am Nat 171:1–9
Smith SA, Montes de Oca AN, Reeder TW, Wiens JJ (2007) A phylogenetic perspective on elevational species richness patterns in Middle American treefrogs: why so few species in lowland tropical rainforests? Evolution 61:1188–1207
Smith CI, Pellmyr O, Althoff DM, Balcazar-Lara M, Leebens-Mack J, Segraves KA (2008) Pattern and timing of diversification in Yucca (Agavaceae): specialized pollination does not escalate rates of diversification. Proc R Soc B Biol Sci 275:249–258
Stebbins GL (1970) Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Ann Rev Ecol Syst 1:307–326
Stebbins GL (1974) Plant species. Evolution above the species level. Harvard University Press, Cambridge, Massachusetts, USA
Steiner KE, Whitehead VB (1988) The association between oil-collecting flowers and oil-collecting bees in the Drakensberg of southern Africa. Monograph Syst Bot Mis Bot Gard 25:259–277
Steiner KE, Whitehead VB (1990) Pollinator adaptation to oil-secreting flowers – Rediviva and Diascia. Evolution 44:1701–1707
Steiner KE, Whitehead VB (1991) Oil flowers and oil bees: further evidence for pollinator adaptation. Evolution 45:1493–1501
Stone GN, Willmer P, Rowe JA (1998) Partitioning of pollinators during flowering in an African Acacia community. Ecology 79:2808–2827
Straw RM (1955) Hybridization, homogamy, and sympatric speciation. Evolution 9:441–444
Straw RM (1956) Floral isolation in Penstemon. Am Nat 90:47–53
Susuki K (1992) Bumblebee pollinators and pollination ecotypes of Isodon umbrosus and I. shikokianus (Lamiaceae). Plant Spec Biol 7:37–48
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago, USA
Thompson JN (2005) The geographic mosaic of coevolutionary. University of Chicago Press, Chicago, USA
Tschapka MS, Dressler S, van Helversen O (2006) Bat visits to Marcgravia pittieri and notes on the inflorescence diversity within the genus Marcgravia (Marcgraviaceae). Flora 201:383–388
van der Niet TS, Johnson SD, Linder HP (2006) Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution 60:1596–1601
Verdu M (2002) Age at maturity and diversification in woody angiosperms. Evolution 56:1352–1361
Waser NM (1983) Competition for pollination and floral character differences among sympatric plant species: a review of the evidence. In: Jones CE, Little RJ (eds) Handbook of experimental pollination ecology. Van Nostrand Reinhold, New York, pp 277–293
Waser NM (1998) Pollination, angiosperm speciation, and the nature of species boundaries. Oikos 82:198–201
Waser NM (2001) Pollinator behavior and plant speciation: looking beyond the “ethological isolation” paradigm. In: Chittka L, Thomson JD (eds) Cognitive ecology of pollination Cambridge University Press, Cambridge, UK, pp 318–335
Waser NM, Campbell DR (2004) Ecological speciation in flowering plants. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) Adaptive speciation. Cambridge University Press, Cambridge, UK, pp 264–277
Waser NM, Chittka L, Price M, Williams N, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Wasserthal LT (1997) The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta 110:343–359
Wege J (1999) Morphological and anatomical variation within Stylidium (Stylidiaceae)––a systematic perspective. PhD Thesis, University of Western Australia, Perth
Whalen MD (1978) Reproductive character displacement and floral diversity in Solanum sect. Androceras. Syst Bot 3:77–86
Whittall JB, Hodges SA (2007). Pollination shifts drive increasingly long nectar spurs in columbine flowers. Nature 447:706–709
Wright S (1940) Breeding structure of populations in relation to speciation. Am Nat 74:232–48
Yang CF, Gituru RW, Guo YH (2007) Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biol J Linnean Soc 90:37–48
Acknowledgments
We thank numerous students, postdoctoral researchers, and collaborators for stimulating discussions and help in the field and glasshouse collecting the morphological and pollination data used in this paper. We thank Roger Härdling, Kathleen Donohue, and two anonymous reviewers for comments on an earlier draft of this paper. Research was funded by the US National Science Foundation (DEB−0324808, DEB-0444745) and the Norwegian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armbruster, W.S., Muchhala, N. Associations between floral specialization and species diversity: cause, effect, or correlation?. Evol Ecol 23, 159–179 (2009). https://doi.org/10.1007/s10682-008-9259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9259-z