Abstract
Solanum commersonii is a wild tuber-bearing species native to Uruguay with high potential for use in potato breeding programs. Little is known about the genetic diversity within this wild species and the relationship with the resistance to the bacterial pathogen Ralstonia solanacearum. We studied 30 S. commersonii clonal accessions, 20 of which were collected from geographically different areas across the country, while the other ten were grown from seeds from a single plant. Resistance against R. solanacearum was tested and different levels of resistance were found, ranging from delayed wilting to asymptomatic reactions. The genetic variation and the relationships among individuals in this germplasm collection were studied by different molecular markers: Random Amplified Polymorphic DNA (RAPD), Amplified Fragment Length Polymorphism (AFLP) and Microsatellites or Simple Sequence Repeats (SSR). AFLP markers generated the largest number of total and polymorphic fragments per assay unit while SSR revealed the highest frequency of polymorphic bands (100%), followed by AFLP (96.2%) and RAPD (89.4%). In contrast, when comparing the number of different genetic profiles generated, the SSR markers exhibited the lowest discriminatory power. The clustering pattern obtained with the three marker systems showed a similar distribution of the S. commersonii germplasm revealing a high correlation between the three methods employed. All three dendrograms grouped most of the accessions into two main clusters, containing the same accessions regardless of the marker type. Bacterial wilt resistant accessions were present in both clusters. Accessions originated from different seeds of the same plant were grouped within one of the major clusters, and differed in the response to R. solanacearum revealing segregation of resistance. Furthermore, the distribution in two main clusters showed high correspondence with the geographical origin of the accessions, from the north and south of the country, and with the subspecies malmeanum and commersonii morphologically identified.
Similar content being viewed by others
Introduction
Knowledge about the genetic variation of wild Solanum species is a pre-requisite for their preservation as well as their use as a germplasm source material in potato (Solanum tuberosum L.) breeding programs. Solanum commersonii Dun is a wild tuber-bearing species native to Uruguay, which is within the centre of its diversity (Hawkes 1990). It possesses many desirable traits, including tolerance to low temperatures and resistance to several pathogens (Tozzini et al. 1991; Chen et al. 1999).
In particular, S. commersonii carries resistance against the bacterial pathogen Ralstonia solanacearum, the causative agent of bacterial wilt (Laferriere et al. 1999). This severe and devastating disease causes extensive damage and significant economic losses to potato production in Uruguay during rainy seasons. R. solanacearum is a soil-borne pathogen that enters the plant through wounds in root tissue and progressively invades the stem vascular tissues, leading to partial or complete wilting and ultimately plant death. It is distributed worldwide, mainly in tropical and subtropical areas, but also in some cool temperate regions (Hayward 1991). It has an unusually wide host range, with over 200 hosts belonging to more than 50 botanical families. Species belonging to the Solanaceae are particularly threatened, including cultivated species such as tomato, potato, eggplant and tobacco (Hayward 1994). R. solanacearum is a complex species with exceptional diversity among strains isolated from different hosts and geographical regions. Traditionally it has been subdivided into five races on the basis of differences in host range (Buddenhagen et al. 1962; Pegg and Moffett 1971) and six biovars on the basis of carbohydrate utilization (Hayward 1964, 1991, 1994). In cool regions bacterial wilt of potato is mainly produced by race 3/biovar 2 strains. This group of strains is very homogeneous, possesses a narrow host range, and is highly virulent mainly towards potatoes and tomatoes. Although assessments of race and biovar have been useful for describing strains worldwide, classification systems based on phenotypic characterization are inherently inconsistent. Fegan and Prior (2005) proposed a hierarchical classification for R. solanacearum, based on phylogenetic analysis of 16S–23S ITS and endoglucanase gene sequences, where race 3/biovar 2 strains belong to Phylotype II, sequevars 1 and 2. This type of strain is predominant in cool and temperate regions of South America, including Uruguay.
Since agrochemicals are costly and largely ineffective, and sanitary cropping systems are sometimes difficult to apply, disease control strategies have so far mostly relied on breeding for resistance (Fock et al. 2001). Some Solanum species have been used as potential source of resistance against R. solanacearum. Unfortunately, sexual hybrids of potato with S. chacoense, S. sparsipillum and S. multidissectum accessions achieved only a moderate level of resistance, together with some undesirable wild traits, such as high glycoalkaloid content (French et al. 1997). Until recently, the use of S. commersonii (2x, 1EBN) in potato breeding (4x, 4EBN) has been scant due to interspecific barriers. Several introgression strategies were proposed exploiting the ability to produce 2n gametes in some clones (Masuelli et al. 1993), chromosome doubling, and in vitro embryo rescue (Jansky 2006). In addition, somatic hybridization between S. commersonii and S. tuberosum was performed, and resistance to R. solanacearum in these hybrids was confirmed (Laferriere et al. 1999; Kim-Lee et al. 2005). Some studies report the utilization of S. commersonii accessions as a source of resistance to bacterial wilt. However, little is known about resistance properties across the whole spectrum of diversity in this wild species. Leaf extracts of several Uruguayan S. commersonii accessions collected in different geographic locations were shown to produce an inhibitory effect on the growth of R. solanacearum suggesting the presence of constitutive compounds associated with resistance (Siri et al. 2005). However, information on the genetic relatedness of these accessions has not been available, and this information is critical since the use of different resistant materials in potato breeding programs may contribute to diversify the genetic bases of the new hybrids and to accumulate desirable resistance alleles in elite germplasm.
A variety of molecular marker systems have been developed, each with specific advantages and disadvantages. The Random Amplified Polymorphic DNA (RAPD) method is quick, easy, and requires no prior sequence information (Williams et al. 1990). The Amplified Fragment Length Polymorphism (AFLP) is a multilocus marker technique developed by Vos et al. (1995). Although the AFLP procedure is more labor intensive and expensive than RAPD analysis, a larger number of loci are assembled in each reaction. Microsatellites or Simple Sequence Repeats (SSR) offer another approach to the detection of polymorphisms in plant genomes (Tautz 1989). The major limitation of this marker system is that it requires prior sequence information for the development of specific primers. Nevertheless, the increasing number of SSR primer sequences published may provide an additional source of markers that can be used to distinguish between closely related taxa.
All three molecular markers systems mentioned above have been used in potato and its wild relatives in order to study genetic diversity, phylogenetic relationships as well as genetic mapping and genotype identification (Ghislain et al. 1999; McGregor et al. 2000; Zmnoch-Guzowska et al. 2000). A better understanding of the usefulness of the different molecular markers for wild species germplasm characterization and classification is considered a priority, and is a prerequisite for more effective breeding programs. It has been shown that different markers may reveal different types of variation. In turn, this is correlated with the genome fraction surveyed by each kind of marker, their distribution throughout the genome and the extent of the DNA target analyzed by each specific assay (Dávila et al. 1999). Therefore, each PCR-based marker system needs careful evaluation before being effectively deployed in this type of analysis. RAPD analysis has been previously used to investigate the biodiversity of accessions collected in a limited area in Uruguay, the southern coastal region. High genetic variation was revealed, even among clones from the same collection point (Pianzzola et al. 2005).
The aim of our research work is to introduce S. commersonii as a potential source of resistance against R. solanacearum. For this purpose we analyzed the genetic diversity of a germplasm collection of S. commersonii by using different PCR-based marker technologies, RAPD, AFLP and SSR. In the case of RAPD markers we extended the S. commersonii population analyzed previously (Pianzzola et al. 2005), and for the first time we used AFLP and SSR techniques to evaluate genetic variation among wild forms of S. commersonii collected from different locations in Uruguay. Finally, we tested these accessions for resistance to R. solanacearum to identify those with the highest potential for breeding purposes and to relate the resistance with the genetic diversity within the species.
Materials and methods
Plant material
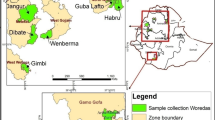
S. commersonii accessions were collected at different sites along the country as vegetative plants or tubers (clones) and seeds (families). This material was maintained and multiplied in vivo in a greenhouse, and seed samples were stored in our genebank. A total of 30 S. commersonii accessions were included in this study (Table 1). Among them, ten accessions originated from different seeds of the same plant (half-sib family, group F) and the other 20 were collected from different locations in Uruguay (groups S and N corresponding to the southern or the northern region of the country, respectively). Plants of S. tuberosum cv. ‘Chieftain’ were used as a susceptible control for the resistance assays, and as outgroup for the genetic analysis.
Molecular analysis
S. commersonii accessions were assessed for molecular diversity using three different molecular markers techniques: RAPD, AFLP and SSR. Genomic DNA was extracted from young leaves using the CTAB (hexadecyltrimethyl ammonium bromide) procedure described by Doyle and Doyle (1987). Accession number 29 was not analyzed by AFLP and SSR due to the insufficient amount of extracted genomic DNA.
RAPD reactions were performed according to the methodology described by Pianzzola et al. (2005) using the Ready-To-Go RAPD Analysis Kit (Amersham-Pharmacia Biotech, USA). Amplification was carried out in a Perkin–Elmer 2400 GeneAmp PCR system. The initial denaturation step at 96°C for 8 min was followed by 35 cycles of 96°C for 1 min, 34°C for 2 min, 72°C for 2 min 30 s and a final extension at 72°C for 10 min. A total of 36 10-mer oligonucleotides randomly chosen from Amersham Pharmacia and Operon Technologies were screened in preliminary experiments in order to evaluate their performance. Of these, the ten that resulted in the most satisfactory amplifications were selected for this study (Table 2). Amplification products (15 μl) were separated on 1.5% (w/v) agarose gels in 1× Tris–borate–EDTA buffer and visualized under UV light after staining with ethidium bromide.
AFLPs were generated according to Vos et al. (1995) with some modifications. Briefly, genomic DNA (250 ng) was double digested with 20 U of EcoRI and 2.5 U of MseI, and double strand adapters were ligated to the fragments ends. Pre-amplification using AFLP +1 primers PE1 (5′-GACTGCGTACCAATTCA-3′) and PM1 (5′-GATGAGTCCTGAGTAAG-3′) was performed in a volume of 25 μl containing 6 pmol of both primers, 0.1 mM of each dNTP, 1× PCR buffer, 1 U of Taq polymerase and 5 μl of the 1/10 diluted ligation mixture. The PCR program started with an initial denaturation at 94°C for 2 min followed by 20 cycles of 94°C for 30 s, 55°C for 60 s, 72°C for 60 s, and a final extension at 72°C for 5 min. Six combinations of selective primer pairs (AFLP +3) were screened and three of them were not included in the final analysis because the amplification profile was consistently too faint to score accurately. The informative primer pairs are listed in Table 2. Each 20 μl PCR reaction contained 5 μl of 1/70 diluted pre-amplification product, 1 pmol of MseI selective primer, 5 pmol of EcoRI selective primer, 1× PCR buffer, 0.16 mM of each dNTP and 1 U of Taq polymerase. All PCR reactions were carried out in a Perkin–Elmer 2400 GeneAmp PCR system. The samples were amplified and prepared for electrophoresis according to the original protocol described by Vos et al. (1995). The PCR products (7 μl) were separated on 6% (w/v) polyacrylamide gel with 7 M urea. Gels ran for 2.5–3 h in 0.5× Tris–borate–EDTA buffer at 90 W constant power in a DNA sequencing unit. The AFLP products were visualized by silver staining as described in the Promega Silver Staining kit instructions.
Four SSR primer pairs described by Milbourne et al. (1998) were used in this study (Table 2). The 16 μl reaction volume contained 5 pmol of each forward and reverse primer, 0.25 mM of each dNTP, 1× PCR buffer, 1 U of Taq polymerase, and 50 ng of template DNA. PCR samples were subjected to the following temperature profile: 7 min denaturation step at 94°C followed by 40 cycles of 45 s at 94°C, 45 s at 55°C, 90 s at 72°C and a final extension step of 5 min at 72°C. Amplification reactions were carried out in a Perkin–Elmer 2400 GeneAmp PCR system. SSR products (5 μl) were separated in 6% (w/v) polyacrylamide gels with 7 M urea. Gels ran for 2.5–3 h in 0.5× Tris–borate–EDTA buffer at 90 W constant power in a DNA sequencing unit. The SSR products were visualized by silver staining as described in the Promega Silver Staining kit.
Data analysis
In order to test the reproducibility of the three marker systems, the RAPD and SSR reactions were repeated at least twice for the 30 accessions of S. commersonii. Due to the relatively large amount of DNA required, the AFLP analyses were only repeated in ten of them. The percentage of reproducibility was determined by dividing the number of reproducible bands by the total number of bands observed. Bands that were not found to be reproducible were counted and not used for the comparative analysis of the techniques. Gel images were analyzed with the software Gel Compar 4.2 (Kortrijk, Belgium). Amplification products were scored for the presence (1) or absence (0) of bands and binary matrices were assembled for each marker system. Similarity matrices were constructed from the binary data with Jaccard’s coefficients and dendograms were generated with the unweighted pair-group method with arithmetic average (UPGMA) clustering algorithm. To check the goodness of fit of the cluster analysis to the associated similarity matrix, cophenetic correlation was computed for all the markers employed. Degree of congruence between different marker types was ascertained using Pearson’s correlation coefficient (Genstat Discovery Edition 4.24, Rothamsted, UK). Profile data was then summarized by 11 marker information indices for each assay unit in order to compare the efficiency of the three marker systems for discrimination between accessions and diversity. An assay unit is defined as one reaction of a specific technique, e.g., one primer for RAPD, one primer combination for AFLP and one primer pair for SSR. Genotype index (GI) was calculated as the average number of genotypes with unique profiles expressed as a fraction of the total accessions fingerprinted (McGregor et al. 2000). Diversity index (DIavp) was calculated as DIavp = ∑(1 − p i 2)/n p, where p i is the frequency of ith allele and n p is the number of polymorphic loci. For dominant markers (RAPDs and AFLPs) it was assumed that each band corresponded to a locus with two alleles, presence or absence of the band (Milbourne et al. 1997). Assay efficiency index (Ai) was calculated as Ai = N e/P, where N e is the total number of effective alleles detected and P is the total number of assays performed for their detection (Pejic et al. 1998).
Assessment of resistance against R. solanacearum
S. commersonii plants in vegetative growing phase with seven to nine expanded leaves were selected for the resistance assays. The species showed a short biological cycle in nature with vegetative phases in spring and fall during ca. 60 days, leading quickly to flowering and plant senescence. This made it difficult to get homogeneous sets of plants between accessions for the resistance tests. Screening assays were performed in a greenhouse and were repeated at least twice in independent experiments. S. tuberosum cv. ‘Chieftain’ replications were included as susceptible control in every test.
A single R. solanacearum strain was used for the screening assays. This strain was isolated from a potato field in San José, Uruguay and was classified as phylotype II/sequevars 1–2 (Siri 2005). The pathogen was frequently re-isolated from wilting plants of S. tuberosum in the screening tests to ensure its pathogenicity. The inoculum was prepared following the methodology described by Thurston and Lozano (1968). Bacterial colonies were grown for 48 h at 28°C on Kelman’s growth medium containing 2,3,5-triphenyl tetrazolium chloride (TTC). A suspension was prepared using 0.9% saline solution, adjusted to a concentration of 1 × 104 cfu ml−1. Inoculations were performed by placing a drop (equivalent to 100 cfu) at the insertion of the third expanded leaf from the top of the plant, and wounding with a needle. Three to five clonal replications were inoculated for each accession. Blank controls were inoculated using saline solution. Plants were covered with individual plastic bags for 48 h after inoculation to ensure conditions conducive to pathogen infection. Wilting symptoms were evaluated several times, from 5 days after inoculation until the end of each test, 18–36 days after inoculation. A severity index was applied from zero (no symptoms) to four (dead plants) (Nielson and Haynes 1960). Finally, the accessions were classified in four categories according to the timing of the reaction in comparison with the susceptible control, as follows: (S) Susceptible, complete wilting simultaneous with the S. tuberosum control; (MS) moderately susceptible, complete wilting delayed with respect to the control; (MR) moderately resistant, incomplete or slight wilting, delayed, not observed in all replicates; (R) Resistant, no symptoms. The data were subjected to the non-parametric statistical comparisons, Kruskal–Wallis and Mann–Whitney signed rank tests, using Genstat Discovery Edition 4.24 (Rothamsted, UK).
Results
Levels of polymorphism
The three marker systems used in this study reliably discriminated between most of the S. commersonii accessions. However, each of the PCR based techniques differed in the type and degree of polymorphism detected. The data generated with each method is summarized in Table 2, and the systems are compared in Table 3.
The total number of assays units varied for each of the marker systems, with ten primers being used for RAPD, three primer combinations for AFLP and four primer pairs for SSR. For RAPD analysis, the ten selected primers produced a total of 179 discrete amplified products of which 160 (89.4%) were polymorphic in at least one pair-wise comparison between S. commersonii accessions. The number of polymorphic bands generated per primer ranged from 10 to 21, with an average frequency of 16. The AFLP analysis was performed using three selective primer combinations and generated a total of 105 bands, of which 101 (96.2%) were polymorphic. The number of polymorphic bands per primer combination ranged from 23 to 41, with an average of 33.7. Primer pairs designed for four SSR loci revealed a total of 20 bands, all of which were polymorphic.
All three assays successfully discriminated between the accessions of S. commersonii and the plants of S. tuberosum used as outgroup. However, each assay differed in its power to discriminate between the S. commersonii accessions. For RAPD analysis, all the primers were able to distinguish between most of the accessions analyzed, with an average of 26.9 distinct genetic profiles per primer. In the case of AFLP, two of the three primer combination used (MseI-G2 + EcoRI-A1 and MseI-G5 + EcoRI-A1) were able to discriminate between all the accessions analyzed, generating unique genetic profiles. The SSR assay had the lowest discriminatory power, generating unique profiles for only ten of the accessions, while seven genotypes represented the other 19 accessions analyzed. In this case, the average number of different genotypes obtained was 7.8 ranging from 3 (loci STM007) to 15 (loci STM102).
Genetic similarity
Table 3 summarizes genetic similarity estimates calculated for each of the marker systems used. Overall, SSR revealed the lowest similarity values and RAPD the highest.
Dendrograms produced from similarity matrices for each marker system are shown in Fig. 1. The clustering pattern obtained with RAPD, AFLP and SSR data showed a similar distribution of the S. commersonii germplasm. All three dendrograms grouped most of the accessions into two main clusters, which comprised the same accessions regardless of the marker type used. The analysis of cophenetic correlations for each method resulted in a very good fit of cophenetic values (0.901 for RAPD, 0.945 for AFLP and 0.910 for SSR), indicating that the dendrograms obtained with the three marker systems are an appropriate representation of their respective similarity matrices. Besides, the correlation coefficients between pairwise combinations of similarity matrices were 0.710 for RAPD—AFLP, 0.746 for RAPD—SSR and 0.741 for AFLP—SSR, revealing a high correspondence between the three marker types.
Comparison of marker usefulness
The results generated by the three marker systems were found to be highly reproducible: 100% for SSR, 94% for AFLP and 92% for RAPD. The lowest level of reproducibility of RAPD was in general agreement with other reports and could probably be explained by the occurrence of competition during amplification (Halldén et al. 1996).
Assay efficiency index (Ai), diversity index (DIavp) and genotype index (GI) were used as estimates for the usefulness of each marker system (Table 3). Overall, information content measured as DIavp was highest for the SSR analysis (0.64), although Ai was highest for AFLP analysis (53.6), followed by RAPD (23.5) and SSR (4.82). When the proportion of different genotypes generated per assay was compared (GI), AFLP showed the best discriminatory power (0.99), followed by RAPD (0.90) and finally SSR (0.27).
Screening for resistance against Ralstonia solanacearum
S. commersonii accessions showed a wide spectrum of reactions after inoculation with R. solanacearum at the insertion of the leaf (Table 1). S. tuberosum control plants began typical wilting symptoms 5–10 days after inoculation in all experiments. S. commersonii accessions were classified in four categories on the basis of the progress of the severity index over time (susceptible, moderately susceptible, moderately resistant, and resistant). A representative evolution of the severity index for the four different categories is shown in Fig. 2.
Disease index evolution in S. commersonii accessions after inoculation with R. solanacearum showing different categories in comparison with the susceptible control S. tuberosum cv. ‘Chieftain’ (S.tub), as follows: susceptible (S), moderately susceptible (MS), moderately resistant (MR) and resistant (R)
Most S. commersonii accessions began wilting at least 1 week later than the control. However, some accessions wilted simultaneously with the control, and therefore those accessions were considered to be susceptible. At the other end of the spectrum, interestingly, for some accessions asymptomatic plants predominated, with delayed and slight symptoms of wilting in only a few replicates. This asymptomatic reaction, so different from that of the control, was observed for the same accessions in independent resistance assays, eliminating the possibility that the plants had not been infected (escapes). Out of the 26 accessions tested, nine were found to be resistant and six moderately resistant. These accessions were present in all phenetic clusters and were collected in different geographic locations in the country, although a higher frequency was found in the southern region (Kruskal–Wallis, P = 0.068).
Discussion
The high level of polymorphism observed in this study for all three marker systems confirmed the high phenotypic diversity that characterizes this wild species and is in agreement with results from previous studies carried out with RAPD markers (Pianzzola et al. 2005).
AFLP analysis generated the greatest number of bands per assay unit, due to the high number of loci identified per assay. However, the highest level of polymorphism was obtained by microsatellite analysis (100%). Comparison of the Diversity Index (DIavp) calculated from the experimental data highlights the high level of polymorphism detectable by SSR in this germplasm set, which is in accordance with the levels of polymorphism revealed by this type of analysis in other comparative studies (Powell et al. 1996; Pejic et al. 1998; Belaj et al. 2002). The hypervariability observed at SSR loci was expected, due to the unique mechanism by which this variation is generated: replication slippage is thought to occur more frequently than single nucleotide mutations and insertion/deletion events, which generate the polymorphisms detectable by RAPD and AFLP analyses (Powell et al. 1996; Milbourne et al. 1997). The observation that AFLP and RAPD analyses yielded very similar DIavp may be a reflection of the way that variation is sampled, and is consistent with results obtained in other plant species (Powell et al. 1996; Milbourne et al. 1997; Garcia-Mas et al. 2000; Belaj et al. 2002). The Assay Efficiency Index (Ai) is of particular interest as it combines the effective number of alleles identified per locus with the number of polymorphic bands detected per assay. For our study, this index allowed us to compare techniques that detect multiple alleles and one or two bands per assay, such as SSR analysis, with techniques that detect two alleles and multiple bands per assay, such as RAPD and AFLP analysis. The highest value of Ai was obtained using the AFLP method. This was due to the simultaneous detection of a large number of polymorphic bands per assay unit by AFLP markers.
The three marker systems used in this study differed in the power of discrimination measured as genotype index (GI) among the germplasm collection of S. commersonii. The AFLP and RAPD techniques were able to discriminate between most of the S. commersonii accessions as expected, as these two marker techniques quickly and effectively target the whole genome. In contrast, as we used a few SSR loci, this marker system generated unique profiles for only ten of the 29 accessions analyzed. Certainly, this may be improved by evaluating additional SSR primers.
Another important factor to consider when evaluating marker efficiency is the ability to determine relationships between accessions based on an estimation of genetic similarity. Genetic similarity coefficients were obtained for all three PCR-derived techniques, reflecting the extreme variability and high resolving power of these methods. The correlation between the similarity matrices generated in this study from different marker techniques was significant. On the basis of UPGMA cluster analysis, a majority of S. commersonii accessions were divided into two distinct groups, which were comprised of the same accessions regardless of marker type (Fig. 1). The three dendrograms differ in the position of the first branch point, 31.5% for SSR, 66.0% for AFLP and 80.6% for RAPD, reflecting the level of polymorphism detected with each system.
Interestingly, the cluster analysis showed a clear association with the geographic origin of the S. commersonii accessions, revealing for the first time by molecular systems that there are at least two different populations in Uruguay, one located in the north (cluster A) and the other in the south (cluster B). Accessions originated from different seeds of a plant collected in the south of the country were grouped within the cluster B, in agreement with the distribution pattern of S. commersonii populations collected along the country. Furthermore, the three marker systems were able to discriminate within this group of accessions, reflecting the genetic variation found in this segregation family.
According to the morphological characteristics of the accessions, these two main phenetic groups defined by the three marker systems may correspond to the two S. commersonii sub-species. Indeed, most accessions from cluster A could be classified as subsp. malmeanum Bitter, with triangular sepals, terminal and lateral leaflets similar in size, and some of them presenting petioles. Accessions from cluster B could be classified as subsp. commersonii Dunal, having oblate acuminated sepals, prominent large terminal leaflet and sessile lateral leaflets (Hawkes 1990). Thus, the three marker systems would agree in their discrimination between intra-specific taxons. S. commersonii subsp. malmeanum has mainly been found in northern Uruguay, although both subspecies have been reported in Rio Grande do Sul (Brazil) (Castro et al. 2007). Two accessions (11 and 14) did not fall within these two major clusters and showed a large degree of marker variation from the rest of the germplasm collection. Morphological evaluation of this plant material indicates that they may represent natural hybrids between S. commersonii and S. chacoense, another tuber-bearing native species of the Solanum genus. Further morphological and genetic analyses are needed in order to confirm this hypothesis.
In contrast to the clear association with the geographic origin mentioned above, bacterial wilt resistance genotypes were present in the two main clusters and spread all over the country. Therefore, a relationship between resistance and genetic variation was not found.
The inoculation method was selected for its effectiveness under our working conditions, as there were no failures of infection in the susceptible control. However, this method is probably more severe than natural infection under field conditions. Variation in resistance among accessions should be confirmed with screening assays using soil inoculation, where the bacteria penetrate through wounds in the underground part of the plant. This may explain the predominant S. commersonii reaction found in this work, which differs from that reported by Laferriere et al. (1999) after inoculation in the soil. In addition, some accessions showed a high frequency of asymptomatic reaction, as recently reported by Carputo et al. (2005) also using leaf inoculation.
Regarding the observed reactions in S. commersonii accessions, two different steps leading to resistance could be distinguished: (1) the disease is stopped at the recognition events of the infection, and (2) the development of symptoms is delayed and reduced. These observations support hypothesis that different mechanisms could be involved in resistance against R. solanacearum in S. commersonii accessions.
Results from this work are encouraging and showed high genetic diversity and resistance to R. solanacearum in this potato related species. Further studies are needed to investigate the genetic basis of bacterial wilt resistance in S. commersonii. The variation observed within the offspring of one plant, a half-sib family, is reflected in different responses to R. solanacearum revealing segregation of resistance. This finding would indicate that more than one gene may be involved, resulting in a spectrum of increasing resistance. Characterization of bacterial wilt resistance in target developed segregating populations will allow us to confirm this hypothesis.
References
Belaj A, Satovic Z, Rallo L, Trujillo I (2002) Genetic diversity and relationships in olive (Olea europaea L.) germplasm collections as determined by randomly amplified polymorphic DNA. Theor Appl Genet 105:638–644
Buddenhagen I, Sequeira L, Kelman A (1962) Designation of races in Pseudomonas solanacearum. Phytopathology 52:726
Carputo D, Alberino S, Espósito N et al (2005) A multidisciplinary approach to introgress resistance to Ralstonia solanacearum into the cultivated genepool. In: Proceedings of the EAPR potato conference, Bilbao
Castro CM, Pereira AS, Costa DM (2007) Wild potato genetic resources conserved in Southern Brazil: current knowledge and future perspectives. Acta Hortic 745:323–330
Chen YKH, Palta JP, Bamberg JB (1999) Freezing tolerance and tuber production in selfed and backcross progenies derived from somatic hybrids between Solanum tuberosum L. and S. commersonii Dun. Theor Appl Genet 99:100–107. doi:10.1007/s001220051213
Dávila JA, Loarce Y, Ferrer E (1999) Molecular characterization and genetic mapping of random amplified microsatellite polymorphism in barley. Theor Appl Genet 98:265–273. doi:10.1007/s001220051067
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Fegan M, Prior P (2005) How complex is the Ralstonia solanacearum species complex. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul
Fock I, Collonnier C et al (2001) Use of Solanum stenotomum for introduction of resistance to bacterial wilt in somatic hybrids of potato. Plant Physiol Biochem 39:899–908. doi:10.1016/S0981-9428(01)01307-9
French ER, Anguiz R, Aley FP (1997) The usefulness of potato resistance to Ralstonia solanacearum for the integrated control of bacterial wilt. In: Prior P, Allen C, Elphinstone J (eds) Bacterial wilt disease molecular and ecological aspects. Springer Verlag, Berlin
Garcia-Mas J, Oliver M, Gómez-Paniagua H, de Vicente MC (2000) Comparing AFLP, RAPD and RFLP markers for measuring genetic diversity in melon. Theor Appl Genet 101:860–864. doi:10.1007/s001220051553
Ghislain M, Zhang D, Fajardo D, Huamán Z, Hijmans RJ (1999) Marker-assisted sampling of the cultivated Andean potato Solanum phureja collection using RAPD markers. Genet Resour Crop Evol 46:547–555. doi:10.1023/A:1008724007888
Halldén C, Hansen M, Nilsson NO, Hjerdin A, Säll T (1996) Competition as a source of errors in RAPD analysis. Theor Appl Genet 93:1185–1192. doi:10.1007/BF00223449
Hawkes JG (1990) The potato: evolution diversity and genetic resources. Belhaven Press, London
Hayward AC (1964) Characteristics of Pseudomonas solanacearum. J Appl Bacteriol 27:265–277
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–89. doi:10.1146/annurev.py.29.090191.000433
Hayward AC (1994) The hosts of Pseudomonas solanacearum. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent Pseudomonas solanacearum. CAB International, Wallingford
Jansky S (2006) Overcoming hybridization barriers in potato. Plant Breed 125:1–12. doi:10.1111/j.1439-0523.2006.01178.x
Kim-Lee H, Moon JS, Hong YS, Kim MS, Cho HM (2005) Bacterial wilt resistance in the progenies of the fusion hybrids between haploid of potato and Solanum commersonii. Am J Potato Res 82:129–137
Laferriere LT, Helgeson JP, Allen C (1999) Fertile S. tuberosum + S. commersonii somatic hybrids as sources of resistance to bacterial wilt caused by R. solanacearum. Theor Appl Genet 98:1272–1278. doi:10.1007/s001220051193
Masuelli RW, Camadro EL, Mendiburu A (1993) 2n gametes in Solanum commersonii Dun and cytological mechanisms of triplandroid formation in triploid hybrids of S commersonii Dun × S. gourlayi Haw. Genome 35:864–869
McGregor CE, Lambert CA, Greyling MM, Louw JH, Warnich L (2000) A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR, AFLP and SSR) in tetraploid potato (Solanum tuberosum L.). Euphytica 113:135–144. doi:10.1023/A:1003925620546
Milbourne D, Meyer RC, Bradshaw JE et al (1997) Comparison of PCR-based marker system for the analysis of genetic relationships in cultivated potato. Mol Breed 3:127–136. doi:10.1023/A:1009633005390
Milbourne D, Meyer RC, Collins AJ et al (1998) Isolation, characterization and mapping of simple sequence repeat loci in potato. Mol Gen Genet 259:233–245. doi:10.1007/s004380050809
Nielson L, Haynes FL (1960) Resistance in Solanum tuberosum to Pseudomonas solanacearum. Am Potato J 37:260–267. doi:10.1007/BF02855800
Pegg K, Moffett M (1971) Host range of the ginger strain of Pseudomonas solanacearum in Queensland. Aust J Exp Agric Anim Husb 11:696–698. doi:10.1071/EA9710696
Pejic I, Ajmone-Marsan P, Morgante M et al (1998) Comparative analysis of genetic similarity among maize inbred lines detected by RFLPs, RAPDs, SSRs, and AFLPs. Theor Appl Genet 97:1248–1255. doi:10.1007/s001220051017
Pianzzola MJ, Zarantonelli L, González G, Franco Fraguas L, Vázquez A (2005) Genetic, phytochemical and biochemical analyses as tools for biodiversity evaluation. Biochem Syst Ecol 33:67–78. doi:10.1016/j.bse.2004.05.012
Powell W, Morgante M, Andre C et al (1996) The utility of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238. doi:10.1007/BF00564200
Siri MI (2005) Estudio del sistema Solanum commersonii—Ralstonia solanacearum enfocado a la búsqueda de marcadores de resistencia. MSc Thesis, Universidad de la República, Montevideo
Siri MI, Villanueva P, Pianzzola MJ et al (2005) In vitro antimicrobial activity of different accessions of Solanum commersonii Dun from Uruguay. Potato Res 47:127–138. doi:10.1007/BF02735979
Tautz D (1989) Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res 17:6469–6471. doi:10.1093/nar/17.16.6463
Thurston HD, Lozano JC (1968) Resistance to bacterial wilt of potatoes in Colombian clones of Solanum phureja. Am Potato J 45:51–55. doi:10.1007/BF02862862
Tozzini AC, Cerioani MF, Saladrigas MV, Hopp HE (1991) Extreme resistance to infection by potato virus × in genotypes of wild tuber-bearing Solanum species. Potato Res 34:317–324. doi:10.1007/BF02360505
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414. doi:10.1093/nar/23.21.4407
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535. doi:10.1093/nar/18.22.6531
Zmnoch-Guzowska E, Marczewski W, Lebecka R et al (2000) QTL analysis of new sources of resistance to Erwinia carotovora ssp. atroseptica in potato done by AFLP, RFLP and resistance-gene-like markers. Crop Sci 40:1156–1167
Acknowledgments
We thank Dr. Patrick Hinrichsen at the Laboratorio de Biotecnología, INIA La Platina, Chile, for kindly allowing Mrs. Siri to perform the AFLP and SSR techniques. This research was supported in part by the Program for the Development of Basic Sciences (PEDECIBA, MSc fellowship) and the Technologic Development Program in Uruguay (PDT 32–24). We thank Dr. Valerie Dee for linguistic revision of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siri, M.I., Galván, G.A., Quirici, L. et al. Molecular marker diversity and bacterial wilt resistance in wild Solanum commersonii accessions from Uruguay. Euphytica 165, 371–382 (2009). https://doi.org/10.1007/s10681-008-9800-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9800-8