Abstract
Fusarium is one of the most important genera of phytopathogenic fungi, causing potato wilt in the field and potato tuber dry rot during storage. The objectives of this study were to identify Fusarium species associated with both potato diseases in different growing regions in Algeria, and to assess their pathogenicity. Among the 152 isolates collected from symptomatic potato plants and tubers in different provinces in Algeria, 13 species of Fusarium and Neocosmospora were identified. Among these three species were isolated only from plants showing symptoms of Fusarium potato wilt (F. oxysporum, F. venenatum, Neocosmospora solani). Two species (F. culmorum, N. tonkinensis) and an isolate of Neocosmospora sp. were found exclusively in tubers with potato dry rot and the remaining ones (F. redolens, F. cf. tricinctum, F. sambucinum, F. cf. incarnatum-equiseti, F. nygamai, F. brachygibbosum and N. falciformis) were associated with both sample types. Fusarium sambucinum was the most frequent species (52.6% of isolates). Fusarium oxysporum and F. nygamai isolates were the most aggressive in the potato wilt pathogenicity test, and F. sambucinum isolates were the most aggressive in the potato tuber pathogenicity test. This is the first study identifying and characterizing potato dry rot and potato wilt pathogens in Algeria.
Similar content being viewed by others
Introduction
Potato (Solanum tuberosum L.) is an important crop plant that has the potential to meet food demand of the fast growing human population. In Algeria, the production of potato was over 4.6 million tons in 2018, with average yield of 31.0 tons / hectare (FAOSTAT 2020). Algeria is ranked among the largest consumer countries of potato, with an annual consumption of more than 111 kg / inhabitant, which in Africa is estimated on average at 4 kg / inhabitant / year.
Potato is prone to many diseases that can ravage the crop before and after harvest. Fungal pathogens cause economic losses in the field, during storage, transport and commercialization (Eken et al. 2000). Fusarium is one of the most important genera of phytopathogenic fungi, causing potato wilt in the field and potato tuber dry rot during storage.
Fusarium dry rot is distributed worldwide and occurs wherever potatoes are grown (Stevenson et al. 2001). According to Cullen et al. (2005), 13 Fusarium species are considered as causal agents of Fusarium dry rot in potatoes worldwide but the species composition depends on the geographic location and the season.
Fusarium sambucinum Fuckel, F. avenaceum (Fries) Sacc, F. culmorum (W. G. Smith) Saccardo, and F. graminearum Schwabe were reported to be pathogenic on potato tubers in Poland (Baturo-Cieśniewska et al. 2015; Stefańczyk et al. 2016). However, F. oxysporum that was not pathogenic on potato tubers in laboratory conditions, was the most frequently isolated from diseased tubers by Stefańczyk et al. (2016). In China F. sambucinum was found to be the predominant species accounting for 56% of the isolates collected by Du et al. (2012). Several Fusarium species have been reported in Iran as causal agents of potato dry rot, with F. sambucinum and F. solani as dominant ones (Esfahani 2005), while in Tunisia, F. solani, F. oxysporum f. sp. tuberosi, F. sambucinum and F. graminearum were most frequent (Daami-Remadi et al. 2006a, b). Recently, some members of the Fusarium solani species complex (FSSC) have been moved to genus Neocosmospora that comprises a separate clade in phylogenetic analyses and contains both plant and human pathogens (Sandoval-Denis and Crous 2018). There is however still more work required in the genus as not all of the species of FSSC have been identified as Neocosmospora and the exact species number in this genus remains vague (Herkert et al. 2019).
Potato dry rot affects both tubers in storage and seed tuber in the field (Wharton et al. 2007). Symptoms of dry rot on tuber appear as small brown lesions that enlarge in all directions after approximately one month of storage. The sinking of the periderm is caused by the inner tissue desiccation which also may lead to a formation of concentric rings of wrinkled skin (Stevenson et al. 2001). The seed tuber can be a source of inoculum (Cullen et al. 2005). Infested soil can be another source. Fusarium spp. can survive for many years as fungal propagules in the soil, as colonizers of living plants or crop debris, as saprophytes, endophytes or facultative pathogens (Burgess et al. 1981). Up to 60% of yield can be lost due to Fusarium dry rot of potato tubers (Secor and Salas 2001).
Fusarium wilt of potato is a vascular disease caused by different Fusarium species, including F. oxysporum f. sp. tuberosi, F. solani and F. sambucinum (Nelson et al. 1981; Daami-Remadi and El Mahjoub 2004). This pathogen infects potato through roots and then colonizes xylem vessels of stems causing necrosis at lower leaves and unilateral leaf yellowing, chlorosis, vascular discoloration, stunting, wilt and eventual death (Hwang and Evans 1985; Kucharek et al. 2000). In Tunisia, Fusarium wilt was reported to cause losses estimated at 30 to 50% of potato yield and decreased tuber quality (Kerkeni et al. 2013).
In addition to economic losses, Fusarium spp. are known to produce mycotoxins that contaminate potato tuber and are toxic to humans and animals (Senter et al. 1991; Bennett and Klich 2003). Mycotoxins can lead to an immune suppressive effect due to their multiple inhibitory properties on eukaryotic cells, including suppressing synthesis of protein, DNA and RNA, restraining of mitochondrial function and affecting cell division and membrane function (Rocha et al. 2005). Most of the Fusarium species are able to produce one or more mycotoxins with various degrees of toxicity (Bottalico and Perrone 2002), often classified as trichothecene and non-trichothecene mycotoxins (Mills 1990). Trichothecenes are produced by F. culmorum, F. graminearum and F. sambucinum (Stępień and Waśkiewicz 2013). An experiment conducted by Xue et al. (2014) revealed that the trichothecenes cumulate not only in the lesion, but also in the adjacent asymptomatic tissue of a potato tuber, which is important from a consumer perspective. Zearalenone, another mycotoxin important due to its common presence and high toxicity, is produced by F. culmorum, F. graminearum, F. cerealis and F. equiseti (Stępień 2014).
Control strategies for Fusarium dry rot include cultural practices such as crop rotation, use of disease free seed, avoiding tuber injuries during harvesting, and reassuring wound healing prior to storage. The scarring zone forms, more or less rapidly depending on the temperature, by suberification of the walls of the cells close to the lesion. Simultaneously, the superficial cells dehydrate (Tivoli et al. 1986). Biological control agents, ultraviolet radiation, as well as chemical control are also used to control the disease (Heltoft et al. 2015). Ayed et al. (2006) reported the high fungicide efficiency in reducing disease incidence of Fusarium wilt of potato.
The objectives of this research were to: (i) identify Fusarium species associated with potato dry rot and wilt in Algeria using combined approaches: restriction profiles of internal transcribed spacer (ITS) sequences, mycotoxin markers and sequencing of chosen conservative genes, and (ii) evaluate their pathogenicity.
Materials and methods
Sampling and isolation of fungal cultures
Mature potato plants with Fusarium wilt symptoms were collected in 2014, 2015 and 2017 from randomly selected fields in central (Algiers: Staouali, Reghaia) and Aïn Defla (Rouina, Arib) provinces in Algeria (Fig. 1). The dry rot samples were collected in storages from tubers harvested in 2014, 2016 and 2017, in central [Tipaza (Sidi-Rached), Aïn Defla (Rouina, Sidi-ouchir and other areas), Blida (Mouzaia), Boumerdes (Bordj-Menaiel), Bouira (AinBessem, El Asnam) and Tiaret], eastern (Skikda), and western (Mostaganem) provinces of Algeria (Fig. 1).
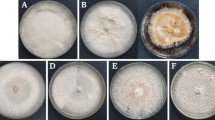
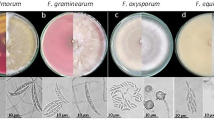
Fungal cultures were isolated from wilted plants and tubers showing symptoms of dry rot. The infected potato roots were washed with tap water to remove all adhering soil particles. Selected organs (rotten tuber fragments, stems fragments, roots and stem bases) were superficially disinfected for 5 min in a 2% sodium hypochlorite solution, rinsed three times with sterile distilled water for 5 min each time and dried with a sterile filter paper. The disinfected fragments 0.5 cm in diameter were transplanted to a PDA (Potato Dextrose Agar) medium, four pieces per plate, and incubated at 25 °C for 5–7 days. Different types of fungal colonies were observed on the PDA medium, but only typical colonies with Fusarium features were selected. Preliminary identification of fungal isolates was done according to the morphological criteria reported by Leslie and Summerell (2006). The main characteristics that were evaluated included macroscopic (colony color and appearance, presence of aerial mycelium) and microscopic traits (presence of chlamydospores and micro- and macroconidia). Subcultures obtained from the colony margin were single-spored by germinating conidia on water agar medium at 16 °C for 1–2 days as described by Stefańczyk and Sobkowiak (2017).
DNA extraction, PCR amplification and sequencing
Single spore cultures were grown in liquid potato sucrose medium at 16 °C for 1 week (Stefańczyk and Sobkowiak 2017). Fungal mycelium was transferred to 1.5 mL Eppendorf tubes, frozen in liquid nitrogen and lyophilized. DNA extractions were performed using GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, St.Louis, MO, USA) according to the manufacturer’s instructions.
The DNA of each isolate along with the DNA of seven control isolates supplied by E. Stefańczyk, listed in the Online Resource 1 and described earlier (Stefańczyk et al. 2016) was used for an amplification of the PCR markers listed in the Table 1. The isolates were scored according to the presence/absence of a product obtained with the mycotoxin markers (Tri5, PKS4 and beas markers, indicating the ability to synthesize trichothecenes, zearalenone and enniatins, respectively) and species-specific markers (FS1, J1A, Fc01, Fg16, CLOX, FE1 and VENB) yielding single band products if template DNA originated from F. sambucinum, F. avenaceum, F. culmorum, F. graminearum, F. oxysporum, F. equiseti or F. venenatum, respectively. The products obtained with the ITS marker were digested in separate reactions using three restriction enzymes. Each restriction mixture consisted of 0.3 μL of an enzyme, 2 μL of a buffer (Tango Yellow for MspI and DraII, Buffer R for TaiI), 7.7 μL of water and 10 μL of ITS product, incubated at 37 °C (MspI and DraII) and 65 °C (TaiI) for three hours. The restriction profiles of ITS sequences were visualized in the 1.5–2% agarose gels stained with ethidium bromide. The results were compared with the profiles obtained in silico for 22 most common Fusarium species (Online Resource 2) using the online tool described by San Millán et al. (2013). The data was used for manual clustering of the fungal isolates into groups corresponding to species by comparing their restriction fragment sizes obtained using three different restriction enzymes. The groups were labelled using different colors in the Online Resource 1 and 2.
To confirm the species identification, products obtained with the TEF marker of at least two representatives from each cluster were sequenced (Online Resource 1). Sequenced ITS fragments were used for identification of fungal isolates belonging to genera different than Fusarium. In case of eight isolates (one F. cf. incarnatum-equiseti, two F. sambucinum and five Neocosmospora spp. isolates), the identification required further support by sequencing the products of bTub and RPB2 markers. The reactions were performed in 20 μL under the conditions presented in the literature (Table 1). The PCR products obtained with the TEF, ITS, bTub and RPB2 primers were purified with a Gen Elute PCR Clean-Up Kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. If more than one band was observed, the product of the expected size was cut out from the gel under the UV light and extracted with a GenElute Gel Extraction Kit (Sigma-Aldrich, St. Louis, MO, USA). DNA sequencing was performed by an external company (IBB PAN, Warsaw, Poland).
Fusarium pathogenicity tests
Fusarium wilt test
Pathogenicity test was carried out on the potato cv. Spunta according to Daami-Remadi and El Mahjoub (2004). In total, 23 isolates were tested; of which 21 isolates belonged to four species of Fusarium obtained from wilted potato plants (Table 3): F. cf. incarnatum-equiseti (seven isolates), F. nygamai (six isolates), F. oxysporum (five isolates) and F. sambucinum (three isolates). Additionally, single isolates of Trichoderma atroviride, and Sarocladium kiliense were tested. Potato seed tubers were planted in plastic pots of 25 cm in diameter, containing a 3:1 mixture of sterile soil and peat. Inoculation, performed about one week after emergence of plants, was done by watering each pot with 100 mL of a spore suspension of the pathogen adjusted to 106 spores mL−1 by using an haemocytometer. Three replications were done to assess each Fusarium isolate’s pathogenicity. Three potato plants were tested in each replication. During the outdoor experiment, air temperatures varied daily between 7 and 22 °C. Plants were grown for 2 months with standard watering.
The following parameters were noted during the experiment: presence of hemiplegic vascular yellowing, number of yellowing or wilting leaves on each stem, and the time of wilting of all inoculated plants. Disease index was evaluated by the estimation of the percentage of yellowing or wilting leaves on each plant (number of diseased leaves divided by the number of all leaves).
Fungal isolations were made from the stem base of each plant and 10 cm above the stem base, in order to re-isolate the pathogen.
Tuber dry rot test
Pathogenicity tests were performed as described by Stefańczyk (2017) on 32 fungal isolates: F. sambucinum (12 isolates), F. oxysporum (5), F. cf. incarnatum-equiseti (5), F. nygamai (3), F. brachygibbosum (2 isolates) and single isolates of: Neocosmospora tonkinensis, F. cf. tricinctum, F. culmorum, Neocosmospora sp. and C. rosea. The test was performed on two separate dates, in two replications on each date. In each replication, five tubers from four Polish potato cultivars (Bartek, Gawin, Hinga, Kuba) were inoculated with fungal isolates. The fifth cultivar Harpun was used in a single replication per date. Pathogenicity of F. brachygibbosum isolates was tested on cvs. Bartek, Harpun, Hinga and Kuba on 20 tubers per cultivar, 80 per isolate in total. The apical end of each tuber was wounded with a sterile metal tool leaving a wound of 10 mm deep and 5 mm wide. Tubers were inoculated by pipetting 50 μL of inoculum into a wound. Plastic trays with inoculated tubers were covered with glass to maintain high air humidity and incubated at 16 °C. After three weeks of incubation, two perpendicular diameters of the lesions were taken from tubers cut in half along their longer axes. The mean of these two diameters represented lesion size of each tuber (Stefańczyk 2017).
Statistical analyses
For tuber dry rot tests results, lesion sizes of each individual tuber were used in the analysis. Data were subjected to analysis of variance (ANOVA) with Statistica version 12. Significance of mean differences in lesion sizes were determined using the Tukey’s test with p = 0.05 as a significance threshold.
To resolve the relationships between fungal isolates, a phylogenetic tree was prepared by a neighbor joining method in the MEGA6 software (Tamura et al. 2013). TEF sequences of chosen 24 isolates from this study and 9 isolates from the studies of Lombard et al. (2019) and Xia et al. (2019) were trimmed and aligned using the ClustalW method. The resulting tree was tested by bootstrapping (1000 replicates). A TEF sequence from the Sarocladium kiliense isolate was used as outgroup.
Results
Identification of Fusarium species
A total of 152 fungal isolates having cultural characters of the genus Fusarium were obtained, of which 99 isolates were recovered from the diseased tubers and 53 isolates from potato plants showing wilt symptoms (23 from roots, 19 from stem bases and 11 from stems) (Table 2). Further identification was performed by clustering the isolates based on results from molecular assays that covered: the ability to synthesize mycotoxins (trichothecenes, zearalenone and enniatins), ITS restriction profiles and Fusarium species-specific primers (Online Resource 1 and 2). Mycotoxin and species-specific markers are illustrated in Online Resource 3. No intraspecific variation in ITS digestion profiles was observed. Of the seven species-specific primers used, CLOX indicating F. oxysporum species, was the most reliable as it gave neither false-positive nor false-negative products. Similar results were obtained for markers specific for F. avenaceum, F. culmorum and F. graminearum (J1A, Fc01 and Fg16, respectively), however in case of these markers, testing for false-negative signals was limited (Online Resource 1). Single false-positive (one isolate of F. sambucinum) and false-negative results were obtained with the FE1 marker diagnostic for F. cf. incarnatum-equiseti. Least reliable were markers specific to closely related species F. sambucinum (two false positives and eight false negatives) and F. venenatum (53 false positives, all obtained within the group of F. sambucinum isolates) (Online Resource 1).
The clustering approach was confirmed by sequencing gene fragments from representatives from each cluster, from 69 isolates in total. The obtained 108 sequences were deposited in the NCBI GenBank (43 ITS, 57 TEF, 6 RPB2 and 2 bTub sequences) and their accession numbers are in the Online Resource 1.
Among the 152 isolates, 142 belonged to genera Fusarium and Neocosmospora, while the remaining 10 isolates were identified as: C. rosea (seven isolates), S. kiliense (two isolates) and T. atroviride (one isolate). Among the 48 Fusarium and Neocosmospora isolates obtained from symptomatic wilting potato plants, 46 isolates were classified as Fusarium and two as Neocosmospora. From symptomatic dry rot tissue, 88 Fusarium and six Neocosmospora isolates were recovered (Table 2). Only five of the Neocosmospora isolates were identified to the species level. For the sixth one (6Aind)A, two markers (TEF, RPB2) were sequenced, but the database searches brought no conclusive results regarding the species.
Sixteen species of fungi were identified (Table 2). Grouping according to the infected host organs revealed a total of 13 species (eight Fusarium spp., two Neocosmospora spp., C. rosea, S. kiliense and T. atroviride) recovered from wilted potato plants and 11 species (seven Fusarium spp. isolates, three Neocosmospora spp. and C. rosea) from potato tubers with dry rot symptoms. Five species (F. oxysporum, F. venenatum, N. solani, but also S. kiliense and T. atroviride) were isolated only from plants with Fusarium wilt symptoms, while 2 species (F. culmorum, N. tonkinensis) and an unidentified isolate of Neocosmospora sp. were obtained exclusively from tubers with potato dry rot. In this study, 8 species were common for both, Fusarium wilt and dry rot (Table 2).
Fusarium nygamai with 13 isolates and, both, F. cf. incarnatum-equiseti and F. oxysporum with 11 isolates each were among the most frequently isolated species from plants with Fusarium wilt symptoms. Dry rot infected potato tubers were dominated by F. sambucinum represented by 75 isolates, followed by eight isolates of F. cf. incarnatum-equiseti (Table 2).
The samples were collected from nine regions of the north of Algeria (Fig. 1): wilted potato plants were derived from Algiers and Aïn Defla (central Algeria), while potato tubers with dry rot symptoms came from Aïn Defla, Blida, Bouira, Bourmerdes, Tipaza and Tiaret (center of Algeria), Mostaganem, (west of Algeria) and Skikda (east of Algeria).
The most diversified Fusarium population was found in Aïn Defla province, where 12 Fusarium species were recorded, of which the species F. cf. incarnatum-equiseti, F. sambucinum, F. nygamai, F. brachygibbosum were common for potato Fusarium wilt and tuber dry rot. Fusarium oxysporum, F. redolens, F. cf. tricinctum and N. solani were associated with potato Fusarium wilt, while Neocosmospora sp., N. falciformis and N. tonkinensis were exclusively found on Fusarium tuber dry rot samples. In Algiers province six species associated with potato Fusarium wilt were identified, with F. oxysporum being dominant (five of 14 isolates). The least frequent were F. venenatum, F. culmorum, Neocosmospora sp. and N. solani, of which single isolates were found on diseased tubers only.
According to data obtained in this study, the most abundant Fusarium species in Algeria, present at seven of nine of the surveyed locations, was F. sambucinum, with 80 isolates that represent 56.3% of all of the Fusarium isolates. Fusarium sambucinum dominated in Tiaret, Blida and Aïn Defla provinces with frequencies of 100, 84.6 and 53.3%, of all isolates, respectively.
Online Resource 1 summarizes also the marker data related to Fusarium species potential abilities to produce trichothecenes, zearalenone and enniatins. PCR assays divided the tested Fusarium species into the five groups. Species unable to produce the tested mycotoxins returned no products with Tri5, PKS4 and beas markers (2/2 F. cf. tricinctum isolates, 8/8 Neocosmospora spp., 1/14 F. nygamai, 3/19 F. cf. incarnatum-equiseti, 4/80 F. sambucinum). Some species were potential producers of trichothecenes only (76/80 F. sambucinum, 2/2 F. brachygibbosum, 1/1 F. venenatum isolate), producers of trichothecenes and zearalenone (5/19 F. cf. incarnatum-equiseti, 1/1 F. culmorum isolate), producers of zearalenone only (11/19 F. cf. incarnatum-equiseti isolates) and producers of enniatins only (13/14 F. nygamai, 11/11 F. oxysporum and 4/4 F. redolens isolates). Variation within three species was observed. Isolates identified as F. sambucinum were mostly producers of trichothecenes only. However, no product with the Tri5 marker was obtained for four F. sambucinum isolates due to either possible loss of the gene and ability to produce trichothecenes, or due to mutation in the primer annealing region resulting in the lack of Tri5 product but not necessary in the lack of trichothecenes production. Fusarium cf. incarnatum-equiseti isolates were classified into three groups: no tested mycotoxin, zearalenone, zearalenone and trichothecenes producers. Among the enniatin-producing F. nygamai isolates, a single one did not yield a product with beas marker (Online Resource 1).
Pathogenicity of Fusarium species
The pathogenicity tests were performed for chosen Fusarium isolates on potato plants (23 isolates) and tubers (32 isolates)
Fusarium wilt test
All 23 isolates used for wilt test originated from plant organs other than tubers. After inoculation with the fungal isolates, the potato plants were observed daily to follow disease progress. The most rapid development of the first symptoms, resulting in hemiplegic yellowing on the base leaves about 13 days after inoculation, was caused by the St1PS, E1St4AC2 and E6PS(27) isolates of F. oxysporum. Further symptoms included growth retardation, wilting of leaves and young stems, as well as upward progress of symptoms (progressive yellowing of the leaves from the base to the top, plant wilting and death). Non-inoculated control plants did not develop any symptoms during the experiment.
No wilting symptoms were observed for plants inoculated with T. atroviride isolate E2ST3DT, F. cf. incarnatum-equiseti isolates E4C(04), R2PS(A) and E4C(06) and F. nygamai isolates E5PS(15) and E9C(32) (Table 3). The foliage of the remaining inoculated plants had withered almost completely by the end of the trial. The percentage of withered leaves per stem (completely withered or showing a symptom of hemiplegia) reached 100% in the case of F. oxysporum isolates E1St4AC2 and St1PS, S. kiliense isolate R3PS(A) and F. nygamai isolate E6C(16). The analysis of variance of the disease index showed significant differences between the fungal isolates tested (Table 3).
Re-isolation from the stems and stem-bases of the inoculated plants resulted in species identical to the ones used for inoculation (data not shown).
Tuber dry rot test
A total of 32 fungal isolates were evaluated for their pathogenicity on potato tubers. While 21 of them originated from tubers with dry rot, 11 were isolates from other plant parts. The ANOVA indicated significant effects of fungal isolate (the strongest effect: 65.9% of variance), potato cultivar (3.7%) and interaction isolate × cultivar (11.7%). Cultivar Bartek was the most resistant with the average lesion size of 11.7 mm and maximum 29.0 mm, followed by cvs. Kuba (average lesion 12.0 mm, maximum 31.5 mm), Gawin (average lesion 13.0 mm, maximum 36.0 mm), Hinga (average lesion 14.7 mm, maximum 43.5 mm) and the most susceptible Harpun (average lesion 14.9 mm, maximum 46.5 mm). To focus on the differences between fungal isolates and species, data of all tests, replications and cultivars was averaged (Table 4). Between the fungal isolates, average lesion sizes ranged from 5 mm to 46.5 mm (Table 4). Lesion sizes less than 13.2 mm did not exceed the size of the mechanical wound significantly and the isolates causing average lesions bigger than 13.2 mm differed from the others according to Tukey’s test (Table 4). Thus, this size was taken as the threshold between pathogenicity and non-pathogenicity. Based on this assumption, pathogenic isolates were found only within F. sambucinum where eight of the tested 12 isolates were pathogenic and caused lesions within range 19.9–22.3 mm. Among them there was an isolate E3ST4AR obtained from root. The MF1 control isolate belonging to the same species caused an average lesion of 24.0 mm.
Discussion
PCR markers for fungal species identification (Table 1) were validated in this study by sequencing the TEF of selected isolates. The PCR markers were reliable and less expensive than sequencing but some may be not useful anymore, due to recent changes within genus Fusarium. Fusarium taxonomy and systematics are changing dynamically (Summerell 2019). Fusarium solani species complex has been moved to genus Neocosmospora (Sandoval-Denis and Crous 2018). Rearrangements have been also proposed in other, like F. oxysporum (Lombard et al. 2019) or F. incarnatum-equiseti species complexes (Xia et al. 2019), from which 21 and 44 phylogenetic species have been extracted. Although the results of identification assays for all 11 isolates described as F. oxysporum in this study were identical, it cannot be excluded that these assays do not have enough resolution to distinguish between the sister species that once formed the complex. Indeed, all the 11 isolates returned a product with the CLOX marker that indicates affinity to F. oxysporum species, but the TEF sequences obtained from two of these isolates were identical to the sequences from F. nirenbergiae (Fig. 2), a species recently extracted from the F. oxysporum species complex based on the combined cmdA, rpb2, tef1 and tub2 sequence alignment (Lombard et al. 2019). Our approach classified 19 isolates to F. incarnatum-equiseti species complex. The obtained TEF sequences were compared with the data from the article of Xia et al. (2019) that disassembled the F. incarnatum-equiseti species complex. Of 12 isolates nested in this complex, nine would be annotated as F. clavum, two as F. caatingaense and one as F. tanahbumbuense (Fig. 2, Online Resource 1). The F. clavum isolates from our study were potential trichothecene and zearalenone or zearalenone alone producers, whereas the isolates of the other two species were unable to synthesize these mycotoxins. Still, the marker approach would be sufficient to distinguish a species complex in general, but development of markers of higher resolution would be required in view of the most recent knowledge.
The neighbor joining tree obtained using the TEF sequences of 33 isolates belonging to the Fusarium and Neocosmospora genera. The tree was tested by bootstrapping (1000 replicates) with a cut-off value of 50%. The species names in square brackets are based on the TEF sequences comparison with data from the studies of species complexes disassembly suggested by Lombard et al. (2019) and Xia et al. (2019). The species name is followed by the isolate name from which a sequence originates and the NCBI GenBank accession number. Isolates with names starting with ‘CBS’ are taken from the Lombard et al. and Xia et al. studies. As outgroup, a TEF sequence from the Sarocladium kiliense isolate was used
Among the 152 isolates collected from symptomatic potato plants and tubers in different provinces in Algeria 13 species of Fusarium and Neocosmospora were identified. Within fungal species isolated from wilted potato F. oxysporum and F. nygamai dominated. According to several studies, F. oxysporum is the most common species causing Fusarium wilt (Tivoli 1988). The number of different species associated with this disease is high compared to the study performed by Daami-Remadi and El Mahjoub (2004) who isolated three species (F. solani, F. sambucinum and F. oxysporum) from plants with potato wilt in Tunisia with F. oxysporum being the most frequent one.
In this study, F. sambucinum was isolated most often from dry rot infected potato tubers, similarly as reported in China (Du et al. 2012) and Egypt (Gherbawy et al. 2019). However, studies done by Song et al. (2008) in South Korea and in Poland (Stefańczyk et al. 2016) showed that F. oxysporum was the predominant species associated with potato dry rot.
Seven of the identified fungal species were detected from both wilting plants and tubers with dry rot symptoms. Among them, two species, F. brachygibbosum and F. nygamai have not been isolated from potato before.
Fusarium brachygibbosum is known to cause various diseases on different hosts, like maize stalk rots, leaf spots on oleander, wilt and die-back of olive and Euphorbia larica (Al-Mahmooli et al. 2013; Mirhosseini et al. 2014; Shan et al. 2017; Trabelsi et al. 2018). The isolates of this species were recovered from tuber as well as from potato roots. In our laboratory pathogenicity test, F. brachygibbosum isolates were not able to induce disease symptoms on potato tubers. The inoculated tubers were however incubated at 16 °C, a temperature that may be too low for F. brachygibbosum, which tends to occur in warmer climate as so far it has been identified in China, Iran, Oman and Tunisia.
Thirteen isolates of F. nygamai were isolated from roots and stems, while only one was extracted from a tuber with dry rot symptoms. Fusarium nygamai was described as a cause of root diseases of rice and Vicia faba, but also of wilting of lentils; similarly to F. brachygibbosum, F. nygamai occurred in countries with warm climate like Italy (Sardinia), Pakistan and Sudan (Balmas et al. 2000; Kurmut et al. 2002; Rauf et al. 2016). Fusarium nygamai is a species genetically closely related to F. oxysporum and F. redolens which is supported by the results of the mycotoxin assay: these three species were producers of only enniatins. Their close relationship is also supported by phylogenetic analyses based on the ITS or TEF gene fragments (Fig. 2). Fusarium nygamai close relationship with F. oxysporum could have an impact on the observed pathogenicity test results: isolates of both species did not form lesions on potato tubers (Table 4), however caused severe wilting symptoms on potato plants (Table 3).
Except for the species of genus Fusarium, some other species were recovered that are associated neither with dry rot nor plants wilting. Recovery of such species may be due to their abundance in the soil or at the phylosphere of potato plants. Some of these fungi can have also beneficial effect on host plants. Trichoderma atroviride is used as a biocontrol agent for a wide range of pathogens that could be applied as an alternative to chemical fungicides (Brunner et al. 2005). Other recovered species, C. rosea, is also described in the literature as biological control agent used against fungal pathogens (Nygren et al. 2018). It was shown to efficiently limit the development of silver scurf caused by Helminthosporium solani in potato (Lysøe et al. 2017). None of these biocontrol agents is used on a wide scale in Algeria.
To gain evidence on role of the identified fungal species as causal agents of potato diseases, pathogenicity tests were performed. Fusarium species isolates from infected plant tissues varied in their pathogenicity, just as in the earlier reports (Ray and Hammerschmidt 1998; El-Hassan et al. 2007). Fusarium nygamai, S. kiliense and F. oxysporum caused the strongest symptoms in wilt test among the eight species tested. That is in accordance with the results of Daami-Remadi and El Mahjoub (2004) who reported that F. oxysporum was the most aggressive among the tested F. solani, F. sambucinum and F. oxysporum.
Dry rot test revealed isolates of F. sambucinum as the most aggressive on potato tubers (Table 4). Similar results were also reported by several studies in the USA (Hanson et al. 1996), in Poland (Stefańczyk et al. 2016) and in Turkey (Aydin et al. 2016; Aydin and İnal 2018). Not all fungi isolated from diseased potato tubers or other plant organs, were able to cause the tuber dry rot or wilt in laboratory conditions. Such isolates may be saprophytes, secondary colonizers or contaminants, as was hypothesized by Stefańczyk et al. (2016). Recent studies revealed that Fusarium spp. can asymptomatically colonize weeds growing among crops. Fusarium spp. were recovered from over 92% of sampled weed plants (Suproniene et al. 2019). In such a way, fungi can survive in an area and infect the crop, when a suitable host species is sown. It is a valid question whether for some Fusarium species a potato plant serves not as a primary, but only as a transient asymptomatic host.
In the present study, a diversity of fungal species isolated from infected potato plants and tubers collected in different regions in Algeria was shown. However, the list of the Fusarium spp. associated with potato in this region is likely still incomplete. The results can be useful for other studies such as the search for resistance against the dominant species associated with both diseases.
References
Al-Mahmooli, I. H., Al-Bahri, Y. S., Al-Sadi, A. M., & Deadman, M. L. (2013). First report of Euphorbia larica dieback caused by Fusarium brachygibbosum in Oman. Plant Disease, 97(5), 687–687.
Aydin, M. H., & İnal, B. (2018). Comparative susceptibility of some commercial potato cultivars to Fusarium sambucinum and F. solani isolates causing tuber dry rot. Applied Ecology and Environmental Research, 16(4), 4879–4892.
Aydin, M. H., Pala, F., & Kaplan, C. (2016). Potato tuber sprout rot caused by Fusarium sambucinum in Turkey. Scientific Papers-Series A-Agronomy, 59, 189–193.
Ayed, F., Daami-Remadi, M., Jabnoun-Khiareddine, H., & El Mahjoub, M. (2006). Potato vascular Fusarium wilt in Tunisia: incidence and biocontrol by Trichoderma spp. Plant Pathology Journal, 5, 92–98.
Balmas, V., Corda, P., Marcello, A., & Bottalico, A. (2000). Fusarium nygamai associated with Fusarium foot rot of rice in Sardinia. Plant Disease, 84(7), 807.
Baturo-Cieśniewska, A., Lenc, L., Grabowski, A., & Lukanowski, A. (2015). Characteristics of Polish isolates of Fusarium sambucinum: molecular identification, pathogenicity, diversity and reaction to control agents. American Journal of Potato Research, 92(1), 49–61.
Bennett, J. W., & Klich, M. (2003). Mycotoxins. Clinical Microbiology Reviews, 16(3), 497–516.
Bottalico, A., & Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology, 108, 611–624.
Brunner, K., Zeilinger, S., Ciliento, R., Woo, S. L., Lorito, M., Kubicek, C. P., & Mach, R. L. (2005). Improvement of the fungal biocontrol agent Trichoderma atroviride to enhance both antagonism and induction of plant systemic disease resistance. Applied and Environmental Microbiology, 71, 3959–3965.
Burgess, L. W., Nelson, P. E., Toussoun, T. A., & Cook, R. J. (1981). General ecology of the Fusaria. In P. E. Nelson, T. A. Toussoun, & R. J. Cook (Eds.), Fusarium: Diseases, Biology and Taxonomy (pp. 225–235). University Park: Pennsylvania State University Press.
Cullen, D. W., Toth, I. K., Pitkin, Y., Boonham, N., Walsh, K., Barker, I., & Lees, A. K. (2005). Use of quantitative molecular diagnostic assays to investigate Fusarium dry rot in potato stocks and soil. Phytopathology, 95, 1462–1471.
Daami-Remadi, M., & El Mahjoub, M. (2004). Emergence en Tunisie de Fusarium oxysporum f. sp. tuberosi agent de flétrissure vasculaire des plants et de pourriture sèche des tubercules de pomme de terre. EPPO Bulletin, 34, 407–411.
Daami-Remadi, M., Jabnoum-Khiareddine, H., Ayed, F., & El Mahdjoub, M. (2006a). Effect of temperature on aggressivity of Tunisian Fusarium species causing potato (Solanum tuberosum L.) tuber dry rot. biocontrol. Journal of Agronomy, 5, 350–355.
Daami-Remadi, M., Jabnoum-Khiareddine, H., Ayed, F., Hibar, K., Znaidi, I. E. A., & El Mahdjoub, M. (2006b). In vitro and in vivo evaluation of individually compost fungi for potato Fusarium dry rot biocontrol. Journal of Biological Sciences, 6, 572–580.
Du, M., Ren, X., Sun, Q., Wang, Y., & Zhang, R. (2012). Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Research, 55(2), 175–184.
Eken, C., Demirci, E., & Șahİn, F. (2000). Pathogenicity of the fungi determined on tubers from potato storages in Erzurum, Türkiye. Journal of Turkish Phytopathology, 29(2/3), 61–69.
El-Hassan, K. I., El-Saman, M. G., Mosa, A. A., & Mostafa, M. H. (2007). Variation among Fusarium spp. the causal of potato tuber dry rot in their pathogenicity and mycotoxins production : Egypt. Journal of Phytopathology, 35(2), 53–68.
Esfahani, M. N. (2005). Susceptibility assessment of potato cultivars to Fusarium dry rot species. Potato Research, 48, 215–226.
FAOSTAT. (2020). http://www.fao.org/faostat/en/#data/QC. Accessed on 04.11.2020.
Gherbawy, Y. A., Hussein, M. A., El Dawy, E. G. A., Hassani, N. A., & Alamri, S. A. (2019). Identification of Fusarium spp. associated with potato tubers in upper Egypt by morphological and molecular characters. Asian Journal of Biochemistry, Genetics and Molecular Biology., 2(3), 1–14.
Hanson, L. E., Schwager, S. J., & Loria, R. (1996). Sensitivity to thiabendazole in Fusarium species associated with dry rot of potato. Phytopathology, 86, 378–384.
Heltoft, P., Molteberg, E. L., Nærstad, R., & Hermansen, A. (2015). Effect of maturity level and potato cultivar on development of Fusarium dry rot in Norway. Potato Research, 58, 205–219. https://doi.org/10.1007/s11540-015-9300-x.
Herkert, P. F., Al-Hatmi, A. M. S., de Oliveira Salvador, G. L., Muro, M. D., Pinheiro, R. L., Nucci, M., Queiroz-Telles, F., Sybren de Hoog, G., & Meis, J. F. (2019). Molecular characterization and antifungal susceptibility of clinical Fusarium species from Brazil. Frontiers in Microbiology, 10, 737. https://doi.org/10.3389/fmicb.2019.00737.
Hwang, S. F., & Evans, I. R. (1985). Eumartii wilt of potato in Alberta. Canadian Plant Disease Survey, 65, 57–59.
Kerkeni, A., Daami-Remadi, M., & Khedher, M. B. (2013). In vivo evaluation of compost extracts for the control of the potato Fusarium wilt caused by Fusarium oxysporum f. sp. tuberosi. The African Journal of Plant Science and Biotechnology, 7, 36–41.
Kucharek T., Jones J.P., Hopkins D., & Strandberg J. (2000). Some diseases of vegetable and agronomic crops caused by Fusarium in Florida. Circular-1025 of Florida Cooperative Extension Service, Institute of Food and Agricultural Science and University of Florida.
Kurmut, A. M., Nirenberg, H. I., Bochow, H., & Buttner, C. (2002). Fusarium nygamai, causal agent of root rot of Vicia faba L. in the Sudan. Mededelingen-Faculteit Landbouwkundige En Toegepaste Biologische Wetenschappen, 67(2), 269–274.
Leslie, J. F., & Summerell, B. A. (2006). The Fusarium laboratory manual. Ames: Blackwell Publishing.
Lombard, L., Sandoval-Denis, M., Lamprecht, S. C., & Crous, P. W. (2019). Epitypification of Fusarium oxysporum – clearing the taxonomic chaos. Persoonia, 43, 1–47.
Lysøe, E., Dees, M. W., & Brurberg, M. B. (2017). A three-way transcriptomic interaction study of a biocontrol agent (Clonostachys rosea), a fungal pathogen (Helminthosporium solani), and a potato host (Solanum tuberosum). Molecular Plant-Microbe Interactions, 30(8), 646–655.
Meng, K., Wang, Y., Yang, P., Luo, H., Bai, Y., Shi, P., Yuan, T., Ma, R., & Yao, B. (2010). Rapid detection and quantification of zearalenone-producing Fusarium species by targeting the zearalenone synthase gene PKS4. Food Control, 21, 207–211.
Mills, J. T. (1990). Mycotoxins and toxigenic fungi on cereal grains in western Canada. Canadian Journal of Physiology and Pharmacology, 68(7), 982–986.
Mirhosseini, H. A., Babaeizad, V., & Hashemi, L. (2014). First report of Fusarium brachygibbosum causing leaf spot on oleander in Iran. Journal of Plant Pathology, 96(2), 431.
Mishra, P. K., Fox, R. T. V., & Culham, A. (2003). Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusaria. FEMS Microbiology Letters, 218, 329–332.
Mulè, G., Susca, A., Stea, G., & Moretti, A. (2003). Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiology Letters, 230, 235–240.
Nelson, P. E., Toussoun, T. A., & Cook, R. J. (Eds.). (1981). Fusarium: Diseases, biology and taxonomy. USA: The Pennsylvania State University Press.
Nicholson, P., Simpson, D. R., Weston, G., Rezanoor, H. N., Lees, A. K., Parry, D. W., & Joyce, D. (1998). Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiological and Molecular Plant Pathology, 53, 17–37.
Nicholson, P., Simpson, D. R., Wilson, A. H., Chandler, E., & Thomsett, M. (2004). Detection and differentiation of trichothecene and enniatin-producing Fusarium species on small-grain cereals. European Journal of Plant Pathology, 110, 503–514.
Nygren, K., Dubey, M., Zapparata, A., Iqbal, M., Tzelepis, G. D., Durling, M. B., Jensen, D. F., & Karlsson, M. (2018). The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evolutionary Applications, 11(6), 931–949.
O’Donnell, K., & Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution, 7, 103–116.
O’Donnell, K., Kistler, H. C., Cigelnik, E., & Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, 95, 2044–2049.
Rauf, C. A., Rafique, K., Naz, F., & Kang, S. (2016). First report of lentil wilt caused by Fusarium nygamai in Pakistan. Journal of Plant Pathology, 98(3), 696.
Ray, H., & Hammerschmidt, R. (1998). Responses of potato tuber to infection by Fusarium sambucinum. Physiological and Molecular Plant Pathology, 53(2), 81–92.
Rocha, O., Ansari, K., & Doohan, F. M. (2005). Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Additives and Contamination, 22, 369–378.
San Millán, R. M., Martínez-Ballesteros, I., Rementeria, A., Garaizar, J., & Bikandi, J. (2013). Online exercise for the design and simulation of PCR and PCR-RFLP experiments. BMC Research Notes, 6, 513.
Sandoval-Denis, M., & Crous, P. W. (2018). Removing chaos from confusion: assigning names to common human and animal pathogens in Neocosmospora. Persoonia., 41, 109–129.
Secor, G. A., & Salas, B. (2001). Fusarium dry rot and Fusarium wilt. In W. R. Stevenson, R. Loria, G. D. Franc, & D. P. Weingartner (Eds.), Compendium of potato diseases (pp. 23–25). St. Paul: APS Press.
Senter, L. H., Sanson, D. R., Corley, D. G., Tempesta, M. S., Rottinghaus, A. A., & Rottinghaus, G. E. (1991). Cytotoxicity of trichothecenes mycotoxins isolated from Fusarium sporotrichioides (MC- 72083) and Fusarium sambucinum in baby hamster kidney (BHK-21) cells. Mycopathologia, 113, 127–131.
Shan, L. Y., Cui, W. Y., Zhang, D. D., Zhang, J., Ma, N. N., Bao, Y. M., Dai, X. F., & Guo, W. (2017). First report of Fusarium brachygibbosum causing maize stalk rot in China. Plant Disease, 101, 837.
Song, H., Lee, H., Jeong, J., Park, H., & Lee, C. (2008). Diversity in beauvericin and enniatins H, I and MK1688 by Fusarium oxysporum isolated from potato. International Journal of Food Microbiology, 122, 296–301.
Stefańczyk, E. (2017). Assessment of potato tuber resistance against dry rot. Plant Breeding and Seed Science., 76, 53–56.
Stefańczyk, E., & Sobkowiak, S. (2017). Isolation, identification and preservation of Fusarium spp. causing dry rot of potato tubers. Plant Breeding and Seed Science, 76, 45–51.
Stefańczyk, E., Sobkowiak, S., Brylińska, M., & Śliwka, J. (2016). Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland. European Journal of Plant Pathology, 145, 871–884.
Stępień, Ł. (2014). The use of Fusarium secondary metabolite biosynthetic genes in chemotypic and phylogenetic studies. Critical Reviews in Microbiology, 40(2), 176–185.
Stępień, Ł., & Waśkiewicz, A. (2013). Sequence divergence of the enniatin synthase gene in relation to production of beauvericin and enniatins in Fusarium species. Toxins, 5(3), 537–555.
Stevenson, W. R., Loria, R., Franc, G. D., & Weingartner, D. P. (2001). Compendium of potato diseases (2nd ed.). St. Paul: The American Phytopathological Society.
Summerell, B. A. (2019). Resolving Fusarium: current status of the genus. Annual Review of Phytopathology, 57, 323–339.
Suproniene, S., Kadziene, G., Irzykowski, W., Sneideris, D., Ivanauskas, A., Sakalauskas, S., & Jedryczka, M. (2019). Asymptomatic weeds are frequently colonised by pathogenic species of Fusarium in cereal-based crop rotations. Weed Research, 59(4), 312–323.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tivoli, B. (1988). Guide d’identification des différentes espèces ou variétés de Fusarium rencontrées en France sur la pomme de terre et dans son environnement. Agronomie, 8, 211–222.
Tivoli, B., Abdul Razzaq, K., Jouan, B., & Lemarchand, E. (1986). Comparative study of the infective potential of different species or varieties of Fusarium, causal agents of dry rot in potato tubers. Potato Research, 29, 12–32.
Trabelsi, R., Gdoura, R., & Triki, M.A. (2018). Fusarium brachygibbosum and Fusarium chlamydosporum causing wilt and die-back of olive in Tunisia. In: A. Kallel, M. Ksibi, H. Ben Dhia, N. Khélifi (eds.) Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. (pp. 581-582). Advances in Science, Technology & Innovation, https://doi.org/10.1007/978-3-319-70548-4_175
Turner, A. S., Lees, A. K., Rezanoor, H. N., & Nicholson, P. (1998). Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathology, 47, 278–288.
Wharton, P., Hammerschmidt, R., & Kirk, W. (2007). Fusarium dry rot. Michigan State University. https://archive.lib.msu.edu/DMC/extension_publications/e2992/e2992.pdf
White, T. J., Bruns, T. D., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal genes form phylogenetics. In M. A. Innis, D. H. Gelfrand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols (Vol. 1990, pp. 315–322). San Diego: Academic Press.
Xia, J. W., Sandoval-Denis, M., Crous, P. W., Zhang, X. G., & Lombard, L. (2019). Numbers to names – restyling the Fusarium incarnatum-equiseti species complex. Persoonia, 43, 186–221.
Xue, H. L., Bi, Y., Tang, Y. M., Zhao, Y., & Wang, Y. (2014). Effect of cultivars, Fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food Chemistry, 151, 236–242.
Yoder, W. T., & Christianson, L. M. (1998). Species-specific primers resolve members of Fusarium section Fusarium: taxonomic status of the edible “Quorn” fungus reevaluated. Fungal Genetics and Biology, 23(1), 68–80.
Zheng, L., Zhao, J., Liang, X., Zhan, G., Jiang, S., & Kang, Z. (2017). Identification of a novel Alternaria alternata strain able to hyperparasitize Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. Frontiers in Microbiology, 8, 71.
Acknowledgments
This work was financed by the National Higher School of Agronomy, Algiers, Algeria, the DGRSDT (La Direction Génerale de la Recherche Scientifique et du Développement Technologique) – Algeria, and at Plant Breeding and Acclimatization Institute-National Research Institute by a statutory dotation 1-3-00-3-05 from the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The research involved neither human participants nor animals.
Informed consent
not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azil, N., Stefańczyk, E., Sobkowiak, S. et al. Identification and pathogenicity of Fusarium spp. associated with tuber dry rot and wilt of potato in Algeria. Eur J Plant Pathol 159, 495–509 (2021). https://doi.org/10.1007/s10658-020-02177-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02177-5