Abstract
Clinical trials on icotinib, a first-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), have shown promising results as targeted therapy for non-small cell lung cancer (NSCLC). This study aimed to establish an effective scoring system to predict the one-year progression-free survival (PFS) of advanced NSCLC patients with EGFR mutations treated with icotinib as targeted therapy. A total of 208 consecutive patients with advanced EGFR-positive NSCLC treated with icotinib were enrolled in this study. Baseline characteristics were collected within 30 days before icotinib treatment. PFS was taken as the primary endpoint and the response rate as the secondary endpoint. Least absolute shrinkage and selection operator (LASSO) regression analysis and Cox proportional hazards regression analysis were used to select the optimal predictors. We evaluated the scoring system using a five-fold cross-validation. PFS events occurred in 175 patients, with a median PFS of 9.9 months (interquartile range, 6.8-14.5). The objective response rate (ORR) was 36.1%, and the disease control rate (DCR) was 67.3%. The final ABC-Score consisted of three predictors: age, bone metastases and carbohydrate antigen 19-9 (CA19-9). Upon comparison of all three factors, the combined ABC-score (area under the curve (AUC)= 0.660) showed a better predictive accuracy than age (AUC = 0.573), bone metastases (AUC = 0.615), and CA19-9 (AUC = 0.608) individually. A five-fold cross-validation showed good discrimination with AUC = 0.623. The ABC-score developed in this study was significantly effective as a prognostic tool for icotinib in advanced NSCLC patients with EGFR mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment with tyrosine kinase inhibitors (TKIs) is strongly recommended for patients with advanced non–small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) mutations that are sensitive to TKIs, such as exon 19 deletion (19-Del) and exon 21 L858R (21-L858R) [1, 2]. It has been shown that EGFR-TKIs can significantly improve the clinical outcomes, including progression-free survival (PFS), disease-free survival (DFS) and overall survival (OS), of EGFR-positive NSCLC patients [3,4,5]. Additionally, compared with standard chemotherapy, EGFR-TKIs displayed higher safety, better tolerability, and patients had improved quality of life when used as the first-line treatment for patients with advanced EGFR-positive NSCLC in previous studies [6,7,8]. Currently, third-generation EGFR-TKIs are in active clinical development, focused on controlling acquired resistance to the targeted therapy. In the past decade, a significant number of patients who followed the sequential treatment approach received first-generation EGFR-TKIs as their initial therapy [9, 10].
Icotinib is an orally ingested first-generation EGFR-TKI with potent antitumour activity and high selectivity [11, 12]. It has proven to be more effective than chemotherapy as the first-line treatment for advanced NSCLC patients with EGFR mutations in a phase III clinical trial [13]. Moreover, icotinib exceeds gefitinib as a second-line or third-line treatment for pretreated patients with advanced NSCLC [14]. Furthermore, it has been widely used in China and there is sufficient evidence of its favorable safety and tolerability profile [15, 16]. Considering the promising results and efficacy of icotinib, this study aimed to investigate an effective prognostic scoring system to predict the one-year PFS for advanced NSCLC patients with EGFR mutations treated with icotinib as an EGFR-TKI targeted therapy.
Serum tumor markers (STMs) and other combined laboratory indexes have been widely used clinically as diagnostic biomarkers and to determine prognosis of cancer patients. In our study, carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA-125), and carbohydrate antigen 19 − 9 (CA19-9) were included due to their marked importance in lung cancer. However, STMs have been reported to present transient changes during cancer therapy, providing insight into the relationship between STMs and tumor progression [17]. Additionally, lung immune prognostic index (LIPI) has been proven to be a useful tool to help select advanced NSCLC patients who can benefit from immune checkpoint inhibitor (ICI) treatment [18]. Moreover, previous studies have indicated that the lymphocyte-monocyte ratio (LMR), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) have vital prognostic value in various kinds of solid tumors, such as gastric cancer and endometrial cancer [19, 20]. Systemic immune-inflammation index (SII) was also shown to be a predominant prognostic factor in patients with NSCLC, [21] gastroesophageal adenocarcinoma, [22] hepatocellular carcinoma, [23] and pancreatic cancer [24]. Our study attempted to select the most effective predictors from all of the above variables to establish a scoring system to predict PFS for EGFR-positive NSCLC patients.
Materials and methods
Patients
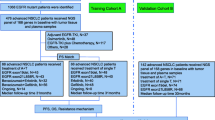
This retrospective clinical study included 208 consecutive patients with advanced EGFR-positive NSCLC treated with icotinib between Januaray 2017 and October 2020 at the Wuhan Union Hospital. Patients were excluded if they did not have laboratory examination results within 30 days prior to the onset of icotinib therapy, or if the follow-up data were missing. Patients received icotinib monotherapy or in combination with other adjuvant treatments, such as chemotherapy and radiotherapy. Patient demographics, tumor characteristics, and laboratory biomarkers including age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking status, tumor histology, EGFR mutation type, tumor stage, metastases, adjuvant treatment, several laboratory combined indices, and three STMs were collected from patients’ medical records. Uncommon EGFR mutations were those other than the exon 19 deletion (19Del), exon 21 L858R (L858R), and compound mutations. The combined indices were calculated as follows: LMR, lymphocyte/monocyte; NLR, neutrophil/lymphocyte; PLR, platelet/lymphocyte; SII, platelet*neutrophil/lymphocyte; prognostic nutritional index (PNI), albumin (g/L) + 5×lymphocyte (109/L); albumin-globulin ratio (AGR), albumin/globulin. LIPI was determined based on the derived neutrophils/(leukocytes minus neutrophils) ratio (dNLR) and level of lactate dehydrogenase (LDH) [18]. The three STMs were CEA, CA-125 and CA19-9.
This study was conducted in accordance with the International Council for Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. The Ethics Committee of Wuhan Union Hospital approved the study protocol and waived the need for informed consent due to the retrospective study design (No. S363).
Assessment of outcomes
The primary endpoint was survival information with PFS and the secondary endpoint was response rate. The best overall response (complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), or not evaluated), objective response rate (ORR\(=\)CR\(+\)PR), and disease control rate (DCR\(=\)CR\(+\)PR\(+\)SD) were evaluated according to the revised Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST ver.1.1) guidelines. PFS was defined as the period from the start of icotinib treatment until disease progression or death. The last follow-up was on August 21, 2021.
Statistical analysis
For baseline characteristics, continuous variables were expressed as a mean ± standard deviation (SD) or median (interquartile range (IQR)), whereas categorical variables were expressed using relative frequencies and proportions. The optimal cut-off values of continuous variables for one-year PFS were identified with the maximal Youden index according to receiver operating characteristic (ROC) curve analysis. The selection of the final prognostic predictors was performed in two steps: [1] Twenty variables, including age, sex, ECOG PS, smoking status, histology, EGFR mutation, disease stage, brain metastases, bone metastases, pleural metastases, LIPI, LMR, NLR, PLR, SII, PNI, AGR, CEA, CA-125, and CA19-9, were enrolled in the least absolute shrinkage and selection operator (LASSO) regression analysis. [2] Thereafter, a Cox proportional hazard model was constructed using the features selected in the LASSO regression model to estimate the hazard ratio (HR) and 95% confidence interval (CI). The final scoring system was validated using a five-fold cross-validation. Survival curves for PFS were plotted using the Kaplan–Meier method. Comparisons of variables between the two groups were performed. As appropriate, the Student’s t-test or the Mann–Whitney U-test was performed for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. A P-value \(<\)0.05 was statistically significant.
Results
Baseline characteristics
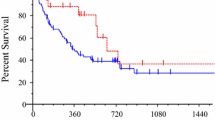
Among the 208 patients, the majority were male (60.1%), non-smoker (74.0%), adenocarcinoma (97.1%), and received adjuvant treatment (60.1%) while taking icotinib (Table 1). Only 9 (4.3%) patients had ECOG PS of 2 or higher, and 18 (8.6%) expressed uncommon EGFR mutations. The mean age was 58.4 years (SD ± 10.5). 51.0% of patients presented with bone metastases, while 31.7% and 29.8% presented with brain and pleural metastases, respectively. PFS events occurred in 175 patients with a median follow-up duration of 19.0 months (range: 9.9–33.3 months) and a median PFS of 9.9 months (IQR: 6.8–14.5). The one-year PFS rate was 55.8% among all patients (Fig. 1). The ORR was 36.1%, and the DCR was 67.3% (Table S1).
LASSO regression analysis
Firstly, the optimal cutoff values of age, LMR, NLR, PLR, SII, PNI, A/G, CEA, CA-125 and CA19-9 for one-year PFS were determined by ROC curve assessment using the Youden index (Table 2). Each continuous variable was converted into two groups based on the optimal cut-off value. Twenty associated characteristic variables were included in the LASSO regression analysis. Seven potential factors, including age, bone metastases, LMR, SII, PNI, CEA, and CA19-9 with nonzero regression coefficients after the shrinkage process, were selected to be most strongly associated with the one-year PFS (Table 3). The LASSO coefficient paths of one-year PFS for all the initial twenty variables and the optimal lambda (λ) are shown in Figure S1.
Selection of the final three prognostic predictors to form the ABC-Scoring system
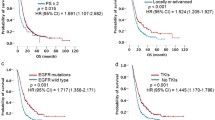
COX regression analysis was performed using the above seven selected variables, and among them, age, bone metastases, and CA19-9 showed significant statistical differences (Table 4). Therefore, the three predictors constituted the ABC-Scoring system to predict the one-year PFS for advanced EGFR-positive NSCLC patients treated with icotinib as EGFR-TKI targeted therapy. Age ≤ 57 were scored as 1, otherwise sored as 0; Having bone metastases were scored as 1, otherwise sored as 0; CA19-9 > 18.4U/ml were scored as 1, otherwise sored as 0. Patients were divided into two groups: the low ABC-Score group (score 0–1) and the high ABC-Score group (score 2–3) (Fig. 2). The one-year PFS rates of patients in the low ABC-Score group and the high ABC-Score group were 55.7% (95%CI: 46.6–64.0%) and 25.7% (95%CI: 16.8–35.6%), respectively. Additionally, the comparison of baseline characteristics between the low and high ABC-Score groups is shown in Table S2. In addition to the three predictors, only disease stage and other tumor metastases showed significant differences between the two ABC-Score groups (P < 0.05). The ORR and DCR of the low and high ABC-Score groups were 37.50% and 35.53%, and 72.12% and 61.85%, respectively.
Predictive performance of ABC-Score
ROC analysis was used to access the predictive performance of the ABC-Score for one-year PFS rate of advanced NSCLC patients treated with icotinib. Results of the analysis showed the following: age (area under the curve (AUC) = 0.573), bone metastases (AUC = 0.615), and CA19-9 (AUC = 0.608). Compared with the three predictors individually, the combined ABC-Score (AUC = 0.660) showed a better predictive accuracy (Fig. 3A). The ABC-Scoring system performed well in the five-fold cross-validation (AUC = 0.623) (Fig. 3B). Kaplan–Meier survival analysis indicated that advanced NSCLC patients in the low ABC-Score group showed better PFS (P < 0.0001) than those in the high ABC-Score group (Fig. 3C). Representative CT images before icotinib treatment, at the time of partial response, and at the time of disease progression in two patients with different ABC-Scores are shown in Fig. 4. The PFS of a 60-year-old woman with an ABC-Score equal to 1 was 17 months, while the PFS of a 57-year-old woman with an ABC-Score of 3 was 8 months.
Example of CT images from pre-treatment to progression of two patients with different ABC-Scores. CT images before icotinib treatment (A), at the time of partial response (B), and at the time of disease progression (C) of a 60-year-old woman with the ABC-Score equal to 1. CT images before icotinib treatment (D), at the time of partial response (E), and at the time of disease progression (F) of a 57-year-old woman with the ABC-Score equal to 3
Subgroup analysis based on adjuvant treatment and EGFR mutation types
Subgroup analysis was performed based on adjuvant treatment and the presence of two common EGFR mutations. It showed that the ABC-Score revealed similar superior predictive performance for one-year PFS for the subgroup with adjuvant treatment (AUC = 0.629) and the subgroup without adjuvant treatment subgroup (AUC = 0.678). There was no significant difference of PFS shown in Kaplan–Meier survival analysis between patients with and without adjuvant treatment (P = 0.9908) (Figure S2). In addition, subgroup analysis of the two types of common EGFR mutations indicated that predictive performance of the ABC-Score was superior for both, the EGFR 19Del subgroup (AUC = 0.679) and the EGFR L858R subgroup (AUC = 0.636). There was no significant difference of PFS noted in the Kaplan–Meier survival analysis between these two subgroups (P = 0.2580) (Figure S3).
Discussion
The median PFS of all the enrolled patients treated with icotinib in our study was 9.9 months, which is similar to previous studies [13, 25]. PD events occurred in 175 NSCLC patients, with 116 events occurring within one year. The primary aim of this study was to select several key predictors and construct a scoring system to determine whether advanced EGFR-positive NSCLC patients have a greater probability for PFS beyond one year with icotinib as EGFR-TKI targeted therapy. Potential variables included patient demographics, tumor characteristics, nutritional and systemic inflammatory combined indices, and serum tumor markers. The final ABC-Score consisted of three predictors: age, bone metastases and CA19-9. In addition, the ROC curves indicated that the scoring system had a better predictive performance than the three predictors alone.
It is universally acknowledged that age is a key risk factor not only for cancer, but many other diseases. While elderly people are commonly considered to have poor healthy conditions, the age ≤ 57 years was a risk factor in our study. It has been reported that younger patients with lung cancer tend to have a worse OS than older group [26]. Moreover, young NSCLC patients are more likely to have advanced stage of disease at diagnosis than older patients [27]. Metastasis is one of the most important features and a major cause of cancer deaths in advanced NSCLC with the advent of diverse extrapulmonary metastatic lesions, among which the most frequent sites are brain, bone and liver [28]. Approximately 40–50% of lung cancer patients have brain metastases, and about 30% of patients simultaneously develop metastasis to the bone when diagnosed with brain metastases from the lung [28, 29]. Patients with lung cancer with liver and bone metastases have been shown to have worse survival than those with other sites of metastasis [28]. In the mean time, previous studies have found that a younger age is an independent risk factor for brain and lymph node metastases in patients with NSCLC [30, 31]. Our study has similar results, with age ≤ 57 years and bone metastasis decreasing the probability for one-year PFS in advanced EGFR-positive NSCLC patients.
One of the enabling characteristics of cancer that has gained authoritative certification is tumor-promoting inflammation, which makes a significant contribution to the activation of core programs in the microenvironment [32]. There is growing evidence that inflammation plays a crucial role in all stages of tumorigenesis and progression. In fact, an increasing number of inflammatory indices and biomarkers have been used to predict the efficacy of immunotherapy and have acted as prognostic factors for cancer patients. Thompson et al. created a weighted score combining epithelial-to-mesenchymal transition (EMT) and inflammatory signatures, which showed high accuracy in predicting responses to ICI therapy in advanced NSCLC patients [33]. Initially, PNI was defined to assess the baseline nutritional status to predict the risk of postoperative complications for malnourished patients with gastrointestinal cancers [34]. Subsequently, PNI level was demonstrated to be associated with prognosis of diverse tumors, tumor stage, and tumor-infiltrating lymphocytes status [35, 36]. Similarly, AGR was shown to be related to OS and lymph node metastasis for cancer patients [37]. In addition, a previous study demonstrated that worsening nutritional status, which was derived from the measures of PNI and body mass index (BMI), indicated poor immunotherapy outcomes for advanced cancer patients [38]. However, none of the combined nutritional and systemic inflammatory indexes enrolled in our study stood out from the statistic analysis.
Although STMs are characterized by low specificity, precise measurement of a panel of STMs can considerably improve the value of early diagnosis and efficacy monitoring of cancers [39]. Another issue is that an increasing of STMs during the disease is closely related to tumour progression. However, changes in STMs within the first four weeks of TKI therapy for advanced NSCLC patients may be unreliable according to Noonan et al [40]. Chen et al. found that preoperative serum CA19-9 could predict the recurrence free survival of patients with lung squamous cell carcinoma [41]. Nevertheless, the pre-treatment level of CA19-9 combined with the other two predictors showed great efficacy to determine the predictive performance of icotinib in this research. More research is needed to confirm the exact changes in STMs that can be considered as signs of tumor progression.
It has been reported that icotinib can easily pass through the cell membrane and blood-brain barrier because of its high permeability to tissue [42]. Liu et al. suggested that pemetrexed combined with icotinib in different sequences had different anticancer capabilities in NSCLC cells, and that treatment with pemetrexed followed by icotinib was the best sequence [43]. Another study demonstrated that icotinib combined with antiangiogenic drugs inhibited tumor growth significantly without increasing the toxicity compared to monotherapy [44]. Additionally, the antiangiogenesis effect was elevated by this combination. Combined therapeutic strategies usually have stronger antitumor effects, owing to the potential effects and interactions between various antitumor drugs. However, the subgroup analysis between the subgroups with and without adjuvant treatment showed no significant differences on PFS. One of the potential reasons for this was the heterogeneity of the detailed patterns of combination treatment with icotinib among patients and the limited size of the cohort in our study. Further exploration about the mechanisms of drug combinations is essential in terms of the complex biological factors and signaling pathways in tumor formation.
The following limitations of this study should be noted. First, it was a single-center retrospective study, which means that the data are less representative. There was no external validation cohort that could be used to further verify the performance of the ABC-Score. Second, information about OS was unavailable due to the long observation period. Therefore, PFS was chosen as the primary endpoint. Third, the heterogeneity of treatment, mainly caused by the concrete chemotherapeutic or radiotherapeutic adjuvant treatment regimens, was not avoided. The comparison of adjuvant treatment between the low and high ABC-Score groups was not statistically significant (P = 0.100). Fourth, this study did not analyze other inflammation-associated markers (such as various immune cells and cytokines) to determine the relationship between inflammation and icotinib efficacy. We plan to improve our research in the future. On the one hand, a multi-center, prospective study is necessary to testify the accuracy of the ABC-Score to predict the efficacy of icotinib among all-stage EGFR-positive NSCLC patients. On the other hand, whether the ABC-Score can be used for predicting the efficacy of other EGFR-TKI-targeted therapies, such as osimertinib, is worthy of future exploration.
To sum up, this study demonstrated that age, bone metastases and CA19-9 can be used to construct an ABC-Score to predict the efficacy of icotinib as an EGFR-TKI targeted therapy for advanced EGFR-positive NSCLC patients. Patients in the low ABC-Score group had a higher probability for PFS beyond one year. Scoring patient before icotinib treatment may influence future therapeutic strategies and guide the efficacy monitoring examinations. Simultaneously, doctors can adopt more individualized strategies according to the precise evaluation of each patient. To validate our results, a prospective study design and external validation cohort are warranted in our future research.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
NCCN clinical practice guidelines In oncology for non-small cell lung cancer. Version 1.2022 (https://www.nccn.org)
Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM et al (2021) Therapy for Stage IV Non-Small-Cell Lung Cancer with driver alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin oncology: official J Am Soc Clin Oncol 39(9):1040–1091
Kelly K, Altorki NK, Eberhardt WE, O’Brien ME, Spigel DR, Crinò L et al (2015) Adjuvant Erlotinib Versus Placebo in patients with Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): a Randomized, Double-Blind, phase III trial. J Clin oncology: official J Am Soc Clin Oncol 33(34):4007–4014
Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y et al (2016) First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.The Cochrane database of systematic reviews. (5):Cd010383
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y et al (2020) Overall survival with Osimertinib in untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382(1):41–50
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222
Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L (2018) Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat reviews Clin Oncol 15(11):694–708
Wu SG, Shih JY (2018) Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 17(1):38
Hu S, Xie G, Zhang DX, Davis C, Long W, Hu Y et al (2012) Synthesis and biological evaluation of crown ether fused quinazoline analogues as potent EGFR inhibitors. Bioorg Med Chem Lett 22(19):6301–6305
Tan F, Shen X, Wang D, Xie G, Zhang X, Ding L et al (2012) Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung cancer (Amsterdam Netherlands) 76(2):177–182
Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP et al (2017) First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 28(10):2443–2450
Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S et al (2013) Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 14(10):953–961
Gu A, Shi C, Xiong L, Chu T, Pei J, Han B (2013) Efficacy and safety evaluation of icotinib in patients with advanced non-small cell lung cancer. Chin J cancer research = Chung-kuo yen cheng yen chiu 25(1):90–94
Shen YW, Zhang XM, Li ST, Lv M, Yang J, Wang F et al (2016) Efficacy and safety of icotinib as first-line therapy in patients with advanced non-small-cell lung cancer. OncoTargets and therapy 9:929–935
Mundle SD, Marathe AS, Chelladurai M (2013) Transient therapy-related surge in serum tumor biomarkers: characterizing behavior and postulating its biologic role. Crit Rev Oncol/Hematol 86(1):15–22
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D et al (2018) Association of the lung Immune Prognostic Index with Immune checkpoint inhibitor outcomes in patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol 4(3):351–357
Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K et al (2015) Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer 113(2):311–320
Xu BB, Xu Y, Lu J, Wu Y, Wang JB, Lin JX et al (2020) Prognostic significance of combined lymphocyte-monocyte ratio and tumor-associated Macrophages in Gastric Cancer Patients after Radical Resection. J Cancer 11(17):5078–5087
Fu F, Deng C, Wen Z, Gao Z, Zhao Y, Han H et al (2021) Systemic immune-inflammation index is a stage-dependent prognostic factor in patients with operable non-small cell lung cancer. Translational lung cancer research 10(7):3144–3154
Jomrich G, Paireder M, Kristo I, Baierl A, Ilhan-Mutlu A, Preusser M et al (2021) High systemic Immune-Inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg 273(3):532–541
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin cancer research: official J Am Association Cancer Res 20(23):6212–6222
Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D et al (2019) The systemic-immune-inflammation index independently predicts survival and recurrence in Resectable Pancreatic Cancer and its Prognostic Value depends on bilirubin levels: a retrospective Multicenter Cohort Study. Ann Surg 270(1):139–146
Li X, Zhang L, Jiang D, Wang Y, Zang A, Ding C et al (2020) Routine-dose and high-dose Icotinib in patients with Advanced Non-Small Cell Lung Cancer Harboring EGFR exon 21-L858R mutation: the Randomized, Phase II, INCREASE Trial. Clin cancer research: official J Am Association Cancer Res 26(13):3162–3171
Shi J, Li D, Liang D, He Y (2021) Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci Rep 11(1):6817
Garrana SH, Dagogo-Jack I, Cobb R, Kuo AH, Mendoza DP, Zhang EW et al (2021) Clinical and Imaging features of non-small-cell Lung Cancer in Young Patients. Clin Lung Cancer 22(1):23–31
Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J et al (2014) Metastatic sites and survival in lung cancer. Lung cancer (Amsterdam Netherlands) 86(1):78–84
Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG (2015) Incidence of brain metastasis at initial presentation of lung cancer. Neurooncology 17(1):122–128
Ji Z, Bi N, Wang J, Hui Z, Xiao Z, Feng Q et al (2014) Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys 89(2):330–337
Xue X, Zang X, Liu Y, Lin D, Jiang T, Gao J et al (2020) Independent risk factors for lymph node metastasis in 2623 patients with non-small cell lung cancer. Surg Oncol 34:256–260
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Thompson JC, Hwang WT, Davis C, Deshpande C, Jeffries S, Rajpurohit Y et al (2020) Gene signatures of tumor inflammation and epithelial-to-mesenchymal transition (EMT) predict responses to immune checkpoint blockade in lung cancer with high accuracy. Lung cancer (Amsterdam Netherlands) 139:1–8
Onodera T, Goseki N, Kosaki G (1984) [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 85(9):1001–1005
Xue Y, Zhou X, Xue L, Zhou R, Luo J (2019) The role of pretreatment prognostic nutritional index in esophageal cancer: a meta-analysis. J Cell Physiol 234(11):19655–19662
Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M et al (2020) Prognostic Nutritional Index, Tumor-infiltrating lymphocytes, and prognosis in patients with esophageal Cancer. Ann Surg 271(4):693–700
Chi J, Xie Q, Jia J, Liu X, Sun J, Chen J et al (2018) Prognostic value of Albumin/Globulin ratio in Survival and Lymph Node Metastasis in patients with Cancer: a systematic review and Meta-analysis. J Cancer 9(13):2341–2348
Johannet P, Sawyers A, Qian Y, Kozloff S, Gulati N, Donnelly D et al (2020) Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer.J Immunother Cancer. ; 8(2)
Gao Y, Huo W, Zhang L, Lian J, Tao W, Song C et al (2019) Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosens Bioelectron 123:204–210
Noonan SA, Patil T, Gao D, King GG, Thibault JR, Lu X et al (2018) Baseline and On-Treatment characteristics of serum tumor markers in Stage IV Oncogene-Addicted Adenocarcinoma of the lung. J Thorac oncology: official publication Int Association Study Lung Cancer 13(1):134–138
Chen H, Fu F, Zhao Y, Wu H, Hu H, Sun Y et al (2021) The prognostic value of preoperative serum tumor markers in Non-Small Cell Lung Cancer varies with Radiological features and histological types. Front Oncol 11:645159
Ni J, Liu DY, Hu B, Li C, Jiang J, Wang HP et al (2015) Relationship between icotinib hydrochloride exposure and clinical outcome in chinese patients with advanced non-small cell lung cancer. Cancer 121(Suppl 17):3146–3156
Liu T, Jin L, Lu W, Gan H, Lin Z, Chen M et al (2019) Sequence-dependent synergistic cytotoxicity of icotinib and pemetrexed in human lung cancer cell lines in vitro and in vivo. J experimental Clin cancer research: CR 38(1):148
Jiang P, Zhang Y, Cui J, Wang X, Li Y (2022) Inhibitory effects of icotinib combined with antiangiogenic drugs in human non-small cell lung cancer xenograft models are better than single target drugs. Thorac cancer 13(2):257–264
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81770096; No. 82070099; No. 82102496; No. 82172034).
Author information
Authors and Affiliations
Contributions
Xueyun Tan: Conceptualization, Project administration, Writing - original draft. Sufei Wang: Investigation, Methodology, Writing - original draft. Hui Xia: Data curation, Investigation, Writing - original draft. Hebing Chen: Formal analysis, Methodology, Data curation. Juanjuan Xu: Funding acquisition, Supervision. Daquan Meng: Data curation, Supervision. Zhihui Wang: Data curation, Investigation, Methodology. Yan Li: Investigation, Supervision. Lian Yang: Data curation, Methodology, Supervision. Yang Jin: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Wuhan Union Hospital approved the study protocols and waived the need for informed consent due to the study design.
Consent for publication
Consent for publication was obtained from all authors.
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xueyun Tan, Sufei Wang, Hui Xia and Hebing Chen contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, X., Wang, S., Xia, H. et al. Prognosis prediction of icotinib as targeted therapy for advanced EGFR-positive non–small cell lung cancer patients. Invest New Drugs 41, 463–472 (2023). https://doi.org/10.1007/s10637-023-01329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01329-8