Summary
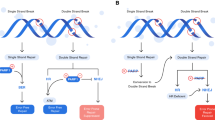
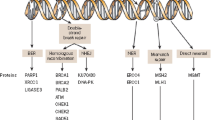
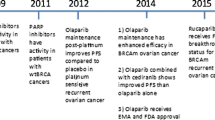
Epithelial ovarian cancer (EOC) accounts for nearly 90% of all ovarian malignancies. The standard therapeutic strategy includes cytoreductive surgery and neo (adjuvant) platinum-based chemotherapy. Relapse of advanced high grade serous ovarian cancer (HGSOC) is related to the development of drug resistance. A defective DNA damage response is a defining hallmark of HGSOC. Poly (ADP-ribose) polymerase (PARP) inhibitors exploit this deficiency through synthetic lethality and have emerged as promising anticancer therapies, especially in breast cancer gene (BRCA1 or BRCA2) mutation carriers. Apart from inducing synthetic lethality, PARP inhibitors have also been shown to trap PARP1 and PARP2 on DNA, leading to PARP-DNA complexes. This “PARP trapping” potentiates synergism between PARP inhibition and both alkylating agents and platinum-based chemotherapy. However, there are remarkable differences in the ability of PARP inhibitors to trap PARP, based on the size and structure of each separate molecule. Since monotherapy with PARP inhibitors is unlikely to induce cancer cell death in BRCA-proficient tumors, the efficacy of PARP inhibitors could be potentially optimized when combined with DNA-damaging agents, or with molecular targeted agents that also impair mechanisms of DNA repair. Olaparib, rucaparib, and niraparib have all obtained US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA) approval in ovarian cancer in different settings. Veliparib does not yet have an approved label; nevertheless, there are currently promising results available in preclinical and early clinical settings. This comprehensive review summarizes the mechanism of action of veliparib and provides an overview of its early and ongoing clinical investigations.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284–296
Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, Ray-Coquard I, Sehouli J, Harter P, du Bois A (2012) The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 23:2605–2612
Jones P, Wilcoxen K, Rowley M, Toniatti C (2015) Niraparib: a poly(ADP-ribose) polymerase (PARP) inhibitor for the treatment of tumors with defective homologous recombination. J Med Chem 58:3302–3314
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434:913–917
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917–921
AlHilli MM, Becker MA, Weroha SJ, Flatten KS, Hurley RM, Harrell MI, Oberg AL, Maurer MJ, Hawthorne KM, Hou X, Harrington SC, McKinstry S, Meng XW, Wilcoxen KM, Kalli KR, Swisher EM, Kaufmann SH, Haluska P (2016) In vivo anti-tumor activity of the PARP inhibitor niraparib in homologous recombination deficient and proficient ovarian carcinoma. Gynecol Oncol 143:379–388
Gogola E, Rottenberg S, Jonkers J (2019) Resistance to PARP inhibitors: lessons from preclinical models of BRCA-associated cancer. Annu Rev Cancer Biol 3:235–254
Boussios S, Karathanasi A, Cooke D, Neille C, Sadauskaite A, Moschetta M, Zakynthinakis-Kyriakou N, Pavlidis N (2019) PARP inhibitors in ovarian cancer: the route to "Ithaca". Diagnostics (Basel) 9:E55
Bridges CB (1922) The origin of variations in sexual and sex-limited characters. Am Nat 56:51–63
Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH (1997) Integrating genetic approaches into the discovery of anticancer drugs. Science 278:1064–1068
Walsh CS (2015) Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol 137:343–350
Valerie K, Povirk LF (2003) Regulation and mechanisms of mammalian double-strand break repair. Oncogene 22:5792–5812
Kraus WL (2015) PARPs and ADP-Ribosylation: 50 years … and counting. Mol Cell 58:902–910
Barkauskaite E, Jankevicius G, Ahel I (2015) Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-Ribosylation. Mol Cell 58:935–946
De Vos M, Schreiber V, Dantzer F (2012) The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol 84:137–146
Farrés J, Llacuna L, Martin-Caballero J, Martínez C, Lozano JJ, Ampurdanés C, López-Contreras AJ, Florensa L, Navarro J, Ottina E, Dantzer F, Schreiber V, Villunger A, Fernández-Capetillo O, Yélamos J (2015) PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ 22:1144–1157
Liu C, Vyas A, Kassab MA, Singh AK, Yu X (2017) The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Res 45:8129–8141
de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A 94:7303–7307
Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9:619–663
Ashworth A (2008) A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26:3785–3790
Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411:366–374
Moschetta M, George A, Kaye SB, Banerjee S (2016) BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol 27:1449–1455
Huang F, Mazin AV (2014) A small molecule inhibitor of human RAD51 potentiates breast cancer cell killing by therapeutic agents in mouse xenografts. PLoS One 9:e100993
Peasland A, Wang LZ, Rowling E, Kyle S, Chen T, Hopkins A, Cliby WA, Sarkaria J, Beale G, Edmondson RJ, Curtin NJ (2011) Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br J Cancer 105:372–381
Bell D, Berchuck A, Birrer M, Chien J, Cramer D, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P et al (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615
Ciardiello F, Bang Y, Bendell J, Cervantes A, Brachmann R, Zhang Y, Raponi M, Farin H, Shen L (2018) A phase 3, double-blind, randomized study of pamiparib versus placebo as maintenance therapy in patients with inoperable, locally advanced, or metastatic gastric cancer that responded to platinum based first-line chemotherapy. Ann Oncol 29:viii205–viii270
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE et al (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13:2728–2737
Pommier Y, O'Connor MJ, de Bono J (2016) Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 8:362ps17
Satoh MS, Lindahl T (1992) Role of poly(ADP-ribose) formation in DNA repair. Nature 356:356–358
Boussios S, Karihtala P, Moschetta M, Karathanasi A, Sadauskaite A, Rassy E, Pavlidis N (2019) Combined strategies with poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian Cancer: a literature review. Diagnostics (Basel) 9:E87
Leo E, Johannes J, Illuzzi G, Zhang A, Hemsley P, Bista MJ, Orme JP, Talbot VA, Narvaez AJ, Underwood E et al (2018) A head-to-head comparison of the properties of five clinical PARP inhibitors identifies new insights that can explain both the observed clinical efficacy and safety profiles. Cancer Res 78:LB–273
Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A et al (2009) Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol 27:2705–2711
Salem AH, Giranda VL, Mostafa NM (2014) Population pharmacokinetic modeling of veliparib (ABT-888) in patients with non-hematologic malignancies. Clin Pharmacokinet 53:479–488
Kikuchi R, Lao Y, Bow DA, Chiou WJ, Andracki ME, Carr RA, Voorman RL, De Morais SM (2013) Prediction of clinical drug-drug interactions of veliparib (ABT-888) with human renal transporters (OAT1, OAT3, OCT2, MATE1, and MATE2K). J Pharm Sci 102:4426–4432
Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, Rubin SC, Rutherford TJ, Chan JK, Chen A et al (2015) A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - an NRG oncology/gynecologic oncology group study. Gynecol Oncol 137:386–391
Murai J (2017) Targeting DNA repair and replication stress in the treatment of ovarian cancer. Int J Clin Oncol 22:619–628
Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y (2014) Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 13:433–443
Puhalla S, Beumer JH, Pahuja S, Appleman LJ, Tawbi HA-H, Stoller RG, Lee JJ, Lin Y, Kiesel B, Yu J et al (2014) Final results of a phase 1 study of single-agent veliparib (V) in patients (pts) with either BRCA1/2-mutated cancer (BRCA+), platinum-refractory ovarian, or basal-like breast cancer (BRCA-wt). J Clin Oncol 32:2570
Steffensen KD, Adimi P, Jakobsen A (2017) Veliparib Monotherapy to patients with BRCA germ line mutation and platinum-resistant or partially platinum-sensitive relapse of epithelial ovarian cancer: a phase I/II study. Int J Gynecol Cancer 27:1842–1849
Ledermann JA (2011) Benefits of enhancing the platinum-free interval in the treatment of relapsed ovarian cancer: more than just a hypothesis? Int J Gynecol Cancer 21:S9–S11
Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, Gandara DR, Allen D, Kiesel B, Beumer JH, Newman EM, Rubinstein L, Chen A, Zhang Y, Wang L, Kinders RJ, Parchment RE, Tomaszewski JE, Doroshow JH (2012) A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res 18:1726–1734
Bell-McGuinn KM, Brady WE, Schilder RJ, Fracasso PM, Moore KN, Walker JL, Duska LR, Mathews CA, Chen A, Shepherd SP et al (2015) A phase I study of continuous veliparib in combination with IV carboplatin/paclitaxel or IV/IP paclitaxel/cisplatin and bevacizumab in newly diagnosed patients with previously untreated epithelial ovarian, fallopian tube, or primary peritoneal cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol 33:5507
Veliparib With Carboplatin and Paclitaxel and as Continuation Maintenance Therapy in Subjects with Newly Diagnosed Stage III or IV, High-grade Serous, Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. (accessed on 22 September 2019); Available online: https://Clinicaltrials.gov/ct2/show/NCT02470585
Landrum LM, Brady WE, Armstrong DK, Moore KN, DiSilvestro PA, O'Malley DM, Tenney ME, Rose PG, Fracasso PM (2016) A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol 140:204–209
Duan W, Gao L, Aguila B, Kalvala A, Otterson GA, Villalona-Calero MA (2014) Fanconi anemia repair pathway dysfunction, a potential therapeutic target in lung cancer. Front Oncol 4:368
Villalona-Calero MA, Duan W, Zhao W, Shilo K, Schaaf LJ, Thurmond J, Westman JA, Marshall J, Xiaobai L, Ji J, Rose J, Lustberg M, Bekaii-Saab T, Chen A, Timmers C (2016) Veliparib alone or in combination with Mitomycin C in patients with solid tumors with functional deficiency in homologous recombination repair. J Natl Cancer Inst 108
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136:823–837
Nishio S, Takekuma M, Takeuchi S, Kawano K, Tsuda N, Tasaki K, Takahashi N, Abe M, Tanaka A, Nagasawa T, Shoji T, Xiong H, Nuthalapati S, Leahy T, Hashiba H, Kiriyama T, Komarnitsky P, Hirashima Y, Ushijima K (2017) Phase 1 study of veliparib with carboplatin and weekly paclitaxel in Japanese patients with newly diagnosed ovarian cancer. Cancer Sci 108:2213–2220
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E et al (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338
Stoller R, Schmitz JC, Ding F, Puhalla S, Belani CP, Appleman L, Lin Y, Jiang Y, Almokadem S, Petro D, Holleran J, Kiesel BF, Ken Czambel R, Carneiro BA, Kontopodis E, Hershberger PA, Rachid M, Chen A, Chu E, Beumer JH (2017) Phase I study of veliparib in combination with gemcitabine. Cancer Chemother Pharmacol 80:631–643
Gray HJ, Bell-McGuinn K, Fleming GF, Cristea M, Xiong H, Sullivan D, Luo Y, McKee MD, Munasinghe W, Martin LP (2018) Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecol Oncol 148:507–514
Wilson RH, Evans TJ, Middleton MR, Molife LR, Spicer J, Dieras V, Roxburgh P, Giordano H, Jaw-Tsai S, Goble S, Plummer R (2017) A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br J Cancer 116:884–892
Wahner Hendrickson AE, Menefee ME, Hartmann LC, Long HJ, Northfelt DW, Reid JM, Boakye-Agyeman F, Kayode O, Flatten KS, Harrell MI, Swisher EM, Poirer GG, Satele D, Allred J, Lensing JL, Chen A, Ji J, Zang Y, Erlichman C, Haluska P, Kaufmann SH (2018) A phase I clinical trial of the poly(ADP-ribose) polymerase inhibitor Veliparib and weekly Topotecan in patients with solid tumors. Clin Cancer Res 24:744–752
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, Villalona-Calero MA, Morgan RJ Jr, Szabo PM, Youn A, Chen AP, Ji J, Allen DE, Lih CJ, Mehaffey MG, Walsh WD, PM MG 3rd, Steinberg SM, Williams PM, Kinders RJ, Conley BA, Simon RM, Doroshow JH (2015) Randomized trial of Oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res 21:1574–1582
Hjortkjær M, Kanstrup H, Jakobsen A, Steffensen KD (2018) Veliparib and topotecan for patients with platinum-resistant or partially platinum-sensitive relapse of epithelial ovarian cancer with BRCA negative or unknown BRCA status. Cancer Treat Res Commun 14:7–12
Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, Jia L, Weil M, Speranza G, Murgo AJ, Kinders R, Wang L, Parchment RE, Carter J, Stotler H, Rubinstein L, Hollingshead M, Melillo G, Pommier Y, Bonner W, Tomaszewski JE, Doroshow JH (2011) Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res 71:5626–5634
Kunos C, Deng W, Dawson D, Lea JS, Zanotti KM, Gray HJ, Bender DP, Guaglianone PP, Carter JS, Moore KN (2015) A phase I-II evaluation of veliparib (NSC #737664), topotecan, and filgrastim or pegfilgrastim in the treatment of persistent or recurrent carcinoma of the uterine cervix: an NRG oncology/gynecologic oncology group study. Int J Gynecol Cancer 25:484–492
LoRusso PM, Li J, Burger A, Heilbrun LK, Sausville EA, Boerner SA, Smith D, Pilat MJ, Zhang J, Tolaney SM, Cleary JM, Chen AP, Rubinstein L, Boerner JL, Bowditch A, Cai D, Bell T, Wolanski A, Marrero AM, Zhang Y, Ji J, Ferry-Galow K, Kinders RJ, Parchment RE, Shapiro GI (2016) Phase I safety, pharmacokinetic, and Pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with Irinotecan in patients with advanced solid tumors. Clin Cancer Res 22:3227–3237
A Trial of ABT-888 in Combination with Temozolomide Versus Pegylated Liposomal Doxorubicin Alone in Ovarian Cancer. (accessed on 22 September 2019); Available online: https://Clinicaltrials.gov/ct2/show/NCT01113957
Chalmers AJ (2004) Poly(ADP-ribose) polymerase-1 and ionizing radiation: sensor, signaller and therapeutic target. Clin Oncol (R Coll Radiol) 16:29–39
Schaefer NG, James E, Wahl RL (2011) Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma. Nucl Med Commun 32:1046–1051
Dungey FA, Löser DA, Chalmers AJ (2008) Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-ribose) polymerase: mechanisms and therapeutic potential. Int J Radiat Oncol Biol Phys 72:1188–1197
Reiss KA, Herman JM, Zahurak M, Brade A, Dawson LA, Scardina A, Joffe C, Petito E, Hacker-Prietz A, Kinders RJ, Wang L, Chen A, Temkin S, Horiba N, Siu L, Azad NS (2015) A phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin Cancer Res 21:68–76
Reiss KA, Herman JM, Armstrong D, Zahurak M, Fyles A, Brade A, Milosevic M, Dawson LA, Scardina A, Fischer P, Hacker-Prietz A, Kinders RJ, Wang L, Chen A, Temkin S, Horiba N, Stayner LA, Siu LL, Azad NS (2017) A final report of a phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid malignancies and peritoneal carcinomatosis with a dose escalation in ovarian and fallopian tube cancers. Gynecol Oncol 144:486–490
Funding
The work was supported by the Medway NHS Foundation Trust, Gillingham, Kent, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Stergios Boussios declares that he has no conflict of interest. Peeter Karihtala declares that he has no conflict of interest. Michele Moschetta declares that he has no conflict of interest. Charlotte Abson declares that she has no conflict of interest. Afroditi Karathanasi declares that she has no conflict of interest. Nikolaos Zakynthinakis-Kyriakou declares that he has no conflict of interest. Jake Edward Ryan declares that he has no conflict of interest. Matin Sheriff declares that he has no conflict of interest. Elie Rassy declares that he has no conflict of interest. Nicholas Pavlidis declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boussios, S., Karihtala, P., Moschetta, M. et al. Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Invest New Drugs 38, 181–193 (2020). https://doi.org/10.1007/s10637-019-00867-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00867-4
Keywords
Profiles
- Stergios Boussios View author profile