Summary
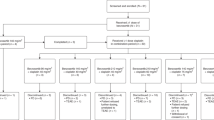
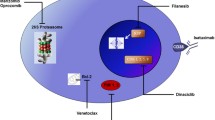
Purpose Filanesib (ARRY-520) is a highly selective, targeted inhibitor of kinesin spindle protein (KSP) inhibitor that induces mitotic arrest and subsequent tumor cell death. This first-in-human Phase 1 study evaluated dose-limiting toxicities (DLTs) and determined a maximum tolerated dose (MTD) for filanesib administered as a 1-h intravenous infusion on 2 treatment schedules in patients with advanced solid tumors. The pharmacokinetics (PK), pharmacodynamics and preliminary efficacy of filanesib were also evaluated. Methods Filanesib was administered on Day 1 of each 3-week cycle (Initial Schedule) or Days 1 and 2 of each 2-week cycle (Alternate Schedule). A standard 3 + 3 dose-escalation design was employed. An expansion cohort was conducted at the MTD of the Initial Schedule. Filanesib PK was evaluated in plasma (both schedules) and urine (Initial Schedule only). Monopolar spindle formation was evaluated in biopsies taken from patients in the expansion cohort. Results Forty-one patients received filanesib. The MTD was equivalent for both the Initial and Alternate Schedules (2.50 mg/m2/cycle). The prevalence of neutropenia as a DLT for both schedules necessitated adding prophylactic filgrastim to another dose escalation on the Alternate Schedule (highest tolerated dose 3.20 mg/m2/cycle). Neurotoxicity related to filanesib was not observed. Dose-proportional increases in filanesib exposure were observed. The half-life for filanesib was ~70 h. Monopolar spindles in patient biopsy samples indicated KSP inhibition. Stable disease was the best tumor response observed in 18 % (7/39) of evaluable patients. Conclusion Filanesib provided exposures with acceptable tolerability and evidence of target-specific pharmacodynamic effects.



Similar content being viewed by others
References
Schmidt M, Bastians H (2007) Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updat 10:162–181. doi:10.1016/j.drup.2007.06.003
Morris PG, Fornier MN (2008) Microtubule active agents: beyond the taxane frontier. Clin Cancer Res 14:7167–7172. doi:10.1158/1078-0432.CCR-08-0169
Janssen A, Medema RH (2011) Mitosis as an anti-cancer target. Oncogene 30:2799–2809. doi:10.1038/onc.2011.30
Sarli V, Giannes A (2008) Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res 14:7583–7587. doi:10.1158/1078-0432.CCR-08-0120
Miglarese MR, Carlson RO (2006) Development of new cancer therapeutic agents targeting mitosis. Expert Opin Investig Drugs 15:1411–1425. doi:10.1517/13543784.15.11.1411
Drummond DR (2011) Regulation of microtubule dynamics by kinesins. Semin Cell Dev Biol 22:927–934. doi:10.1016/j.semcdb.2011.09.021
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288:88–95. doi:10.1126/science.288.5463.88
Blangy A, Lane HA, d’Hérin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83:1159–1169. doi:10.1016/0092-8674(95)90142-6
Stern BM, Murray AW (2001) Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr Biol 11:1462–1467. doi:10.1016/S0960-9822(01)00451-1
Vijapurkar U, Wang W, Herbst R (2007) Potentiation of kinesin spindle protein inhibitor-induced cell death by modulation of mitochondrial and death receptor apoptotic pathways. Cancer Res 67:237–245. doi:10.1158/0008-5472.CAN-06-2406
Wojcik EJ, Buckley RS, Richard J, Liu L, Huckaba TM, Kim S (2013) Kinesin-5: cross-bridging mechanism to targeted clinical therapy. Gene 531:133–149. doi:10.1016/j.gene.2013.08.004
Sakowicz R, Finer JT, Beraud C, Crompton A, Lewis E, Fritsch A et al (2004) Antitumor activity of a kinesin inhibitor. Cancer Res 64:3276–280. doi:10.1158/0008-5472.CAN-03-3839
Huszar D, Theoclitou ME, Skolnik J, Herbst R (2009) Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev 28:197–208. doi:10.1007/s10555-009-9185-8
El-Nassan HB (2013) Advances in the discovery of kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur J Med Chem 62:614–631. doi:10.1016/j.ejmech.2013.01.031
Woessner R, Tunquist B, Lemieux C, Chlipala E, Jackinsky S, Dewolf W Jr et al (2009) ARRY-520, a novel KSP inhibitor with potent activity in hematological and taxane-resistant tumor models. Anticancer Res 29:4373–4380
Woessner RD, Corrette C, Allen S, Hans J, Zhao Q, Aicher T et al (2007) ARRY-520, a KSP inhibitor with efficacy and pharmacodynamic activity in animal models of solid tumors. Proc Am Assoc Cancer Res 48:1433
Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL et al (2002) Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 100:194–199. doi:10.1182/blood.V100.1.194
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671–676. doi:10.1038/nature02067
Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K et al (2005) Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307:1101–1104. doi:10.1126/science.1106114
Zhang B, Gojo I, Fenton RG (2002) Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 99:1885–1893. doi:10.1182/blood.V99.6.1885
Tunquist BJ, Woessner RD, Walker DH (2010) Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther 9:2046–2056. doi:10.1158/1535-7163.MCT-10-0033
Lonial S, Shah JJ, Zonder J, Bensinger WI, Cohen AD, Kaufman JL et al (2013) Prolonged survival and improved response rates with ARRY-520 in relapsed/refractory multiple myeloma (RRMM) patients with low α-1 acid glycoprotein (AAG) levels: results from a Phase 2 study. Blood 122:285
Shah JJ, Feng L, Thomas SK, Weber DM, Wang M, Hilder B et al (2013) Phase 1 Study of the novel kinesin spindle protein inhibitor ARRY-520 + carfilzomib (Car) in patients with relapsed and/or refractory multiple myeloma (RRMM). Blood 122:1982
Chari A, Htut M, Zonder J, Fay JW, Jakubowiak AJ, Harrison B et al (2013) A Phase 1 study of ARRY-520 with bortezomib (BTZ) and dexamethasone (dex) in relapsed or refractory multiple myeloma (RRMM). Blood 122:1938
Chari A, Htut M, Zonder J, Fay JW, Jakubowiak AJ, Harrison B et al. (2014) Phase 1 study of filanesib (ARRY-520) with bortezomib (BTZ) and dexamethasone (dex) in relapsed or refractory multiple myeloma (RRMM). Presented at the 19th Congress of European Hematology Association (EHA), 06 June 2014, Milan, Italy. P370 (abstract)
Acknowledgments
Medical writing support was provided by Allison L. Marlow and Sara Symons. Additional clinical pharmacology support was provided by Burgess Freeman, Sharon Karan, Jason Neale and Micaela Reddy. Additional translational medicine support was provided by Duncan Walker and Brian Tunquist. The authors thank the participating patients, their families, study Investigators, the clinic nurses and the study coordinators at both institutions for their invaluable contributions.
Compliance with ethical standards
ᅘ
Disclosure of potential conflicts of interest
K. Litwiler, M. Ptaszynski, D. Roseberry, S. Rush, J. Schreiber and H.M. Simmons are current or former employees of Array BioPharma Inc. and hold or have held stock and/or stock options in Array BioPharma Inc. P.M. LoRusso, E.A. Sausville, P.H. Goncalves, L. Casetta and J.A. Carter declare that they have no conflicts of interest.
Funding
This study was funded by Array BioPharma, Inc., Boulder, CO.
Ethical standards
This study was conducted in accordance with applicable ICH Good Clinical Practice guidelines and was approved by the institutional review boards of all participating sites. Written informed consent was obtained from all individual participants included in the study.
Prior presentations
Preliminary results presented in poster format at the 2010 American Society of Clinical Oncology Annual Meeting; Chicago, Illinois, USA; 4–8 June 2010. J Clin Oncol 2010;28:15 s(suppl; abstr 2570).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LoRusso, P.M., Goncalves, P.H., Casetta, L. et al. First-in-human phase 1 study of filanesib (ARRY-520), a kinesin spindle protein inhibitor, in patients with advanced solid tumors. Invest New Drugs 33, 440–449 (2015). https://doi.org/10.1007/s10637-015-0211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0211-0