Summary
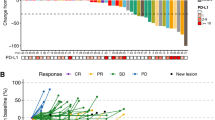
Purpose Preclinical studies in human melanoma cell lines and murine xenograft tumor models suggest that the proteasome inhibitor bortezomib enhances the activity of the cytotoxic agent dacarbazine. We performed a phase I trial of bortezomib and dacarbazine in melanoma, soft tissue sarcoma, and amine precursor uptake and decarboxylation tumors. The primary objective was to identify recommended phase II doses for the combination. Experimental design Bortezomib and dacarbazine were both administered intravenously once weekly. All patients received prophylactic antiemetics. Dose escalation proceeded using a standard 3 + 3 design. Response was assessed according to NCI RECIST v1.0. Results Twenty eight patients were enrolled to six dose levels. Bortezomib 1.6 mg/m2 and dacarbazine 580 mg/m2 are the recommended phase II weekly doses. The combination was generally well tolerated. Among 15 patients with melanoma there was one durable complete response in a patient with an exon-11 cKIT mutation, and one partial response. Among 12 patients with soft tissue sarcoma there was one partial response. Conclusions Bortezomib 1.6 mg/m2 and dacarbazine 580 mg/m2 administered intravenously once weekly is well tolerated and has at least minimal activity in melanoma and soft tissue sarcoma.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S et al (2000) Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 18:158–166
McConkey DJ, Zhu K (2008) Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat 11:164–179
Amiri K, Richmond A (2003) The novel proteasome inhibitor bortezomib (VELCADE), formerly known as PS-341, inhibited growth and overcame drug resistance in human malignant melanoma in vitro and in mice. Proc AACR 44:165
Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A (2004) Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res 64:4912–4918
Kane RC, Farrell AT, Sridhara R, Pazdur R (2006) United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 12:2955–2960
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, BRIM-3 Study Group (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516
Robert C, Thomas L, Bondarenko I, O’Day S, JW MD, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526
Adams J (2004) The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5:417–421
Lawley PD, Phillips DH (1996) DNA adducts from chemotherapeutic agents. Mutat 355:13–40
Millennium Pharmaceuticals, Inc (2012) VELCADE (bortezomib) for injection prescribing information
Hopsira, Inc. (2007) Dacarbazine for injection prescribing information
Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR (2002) A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8:2505–2511
Brennan MF, Alektiar KM, Maki RG (2001) Soft tissue sarcoma. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: Principles and practice of oncology. Lippincott Williams & Williams, pp1841–1891
Moertel CG (1983) Treatment of carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol 1:787–40
Su Y, Amiri KI, Horton LW, Yu Y, Ayers GD, Koehler E et al (2010) A phase I trial of bortezomib with temozolomide in patients with advanced melanoma: toxicities, antitumor effects, and modulation of therapeutic targets. Clin Cancer Res 16:348–357
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ (2012) CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 18:2039–2047
Grossmann A, Grossmann K, Wallander M (2012) Molecular testing in malignant melanoma. Diagn Cytopathol 40:503–510
Bauer S, Parry JA, Mühlenberg T, Brown MF, Seneviratne D, Chatterjee P, Chin A, Rubin BP, Kuan SF, Fletcher JA, Duensing S, Duensing A (2010) Proapoptotic activity of bortezomib in gastrointestinal stromal tumor cells. Cancer Res 70:150–159
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lin X, Du N, Zhang X, Li J, Wang B, Qin S (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29:2904–2909
Enzler T, Sano Y, Choo MK, Cottam HB, Karin M, Tsao H, Park JM (2011) Cell-selective inhibition of NF-κB signaling improves therapeutic index in a melanoma chemotherapy model. Cancer Dis 1:496–507
Li C, Chen S, Yue P, Deng X, Lonial S, Khuri FR, Sun SY (2010) Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IkappaB(alpha) degradation. J Biol Chem 285:16096–16104
Acknowledgments
Supported in part by P30CA016059 (NCI), M01RR000065 (NCRR), and a grant from Millennium Pharmaceuticals, Inc.
Conflict of interest disclosures
The authors have no relevant conflicts to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poklepovic, A., Youseffian, L., Winning, M. et al. Phase I trial of bortezomib and dacarbazine in melanoma and soft tissue sarcoma. Invest New Drugs 31, 937–942 (2013). https://doi.org/10.1007/s10637-012-9913-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9913-8