Abstract
Purpose
To investigate the center-periphery distribution of ON and OFF retinal responses in complete congenital stationary night blindness (cCSNB).
Methods
Photopic full-field flash ERGs (photopic ffERGs) and OPs (photopic ffOPs) and slow m-sequence (to enhance OP prominence) mfERGs (and filtered mfOPs) evoked by a 37 hexagon stimulus array were recorded from normal subjects and cCSNB patients. Discrete wavelet transform (DWT) analysis of photopic ffERGs and mfERGs was also performed in order to assess the contribution of the ON and OFF retinal pathways (i.e., OFF-to-ON ratio) in both cohorts.
Results
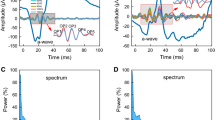
As expected, the photopic ffERG (and ffOPs) responses in cCSNB were devoid of the first two of the three OPs (i.e., OP2 and OP3 and OP4) normally seen on the ascending limb of the b-wave. A similar finding was also noted in the mfERGs (and mfOPs) of ring 4. In contrast, the mfERGs (and mfOPs) of ring 1 included all three OPs. DWT analysis revealed that while in normal subjects, the OFF-to-ON ratio of mfERGs slightly increased from rings 1 to 4 (from 0.61 ± 0.03 to 0.78 ± 0.04; p < 0.05; median: from 0.62 to 0.79; p < 0.05), in cCSNB this ratio increased significantly more [from 0.73 ± 0.13 (ring 1) to 1.18 ± 0.17 (ring 4); p < 0.05; median: 0.78 to 1.22; p < 0.05], hence from a normal ON-dominated ratio (central ring) to an OFF-dominated ratio (peripheral ring).
Conclusions
Our results show a clear discrepancy of ON and OFF mfOP components in cCSNB. Responses originating from the most central ring (i.e., ring 1) disclosed a near-normal electrophysiological contribution (as revealed with the presence of OP2, OP3 and OP4 as well as with the DWT OFF-to-ON ratio) of the retinal ON and OFF pathways in mfERG (and mfOPs) responses compared to responses from the more peripheral ring (and ffOP) which are devoid of the ON OPs (i.e., OP2 and OP3).





Similar content being viewed by others
References
Der Kaloustian VM, Baghdassarian SA (1972) The autosomal recessive variety of congenital stationary night-blindness with myopia. J Med Genet 9(1):67–69
Miyake Y (ed) (2006) Complete and incomplete types of CSNB. In: Electrodiagnosis of retinal disease, chap 2.10. Springer, Tokyo, pp 90–113
Carr RE et al (1966) Rhodopsin and the electrical activity of the retina in congenital night blindness. Invest Ophthalmol 5(5):497–507
Heckenlively JR, Martin DA, Rosenbaum AL (1983) Loss of electroretinographic oscillatory potentials, optic atrophy, and dysplasia in congenital stationary night blindness. Am J Ophthalmol 96(4):526–534
Lachapelle P, Little JM, Polomeno RC (1983) The photopic electroretinogram in congenital stationary night blindness with myopia. Invest Ophthalmol Vis Sci 24(4):442–450
Quigley M et al (1996) On- and off-responses in the photopic electroretinogram in complete-type congenital stationary night blindness. Doc Ophthalmol 92(3):159–165
Schuster A et al (2005) Multifocal oscillatory potentials in CSNB1 and CSNB2 type congenital stationary night blindness. Int J Mol Med 15(1):159–167
Kondo M et al (2008) Comparison of focal macular cone ERGs in complete-type congenital stationary night blindness and APB-treated monkeys. Vis Res 48(2):273–280
Tremblay F, Parkinson J (2008) Gradient of deficit in cone responses in the incomplete form of congenital stationary night blindness revealed by multifocal electroretinography. Doc Ophthalmol 116(1):41–47
Lachapelle P et al (1993) Recording the oscillatory potentials of the electroretinogram with the DTL electrode. Doc Ophthalmol 83(2):119–130
McCulloch DL et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Hood DC et al (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124(1):1–13
Hood DC et al (1997) A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci 14(3):533–544
Rangaswamy NV et al (2006) Effect of experimental glaucoma in primates on oscillatory potentials of the slow-sequence mfERG. Invest Ophthalmol Vis Sci 47(2):753–767
Chakor H et al (2005) Evidence suggesting a center to periphery organisation of multifocal ERG oscillatory potentials (mfOPs). Invest Ophthalmol Vis Sci 46:3427
Chakor AD et al (2004) Are multifocal OPs (mfOPs) equivalent to flash OPs (FOPs)? Invest Ophthalmol Vis Sci 46:3427
Wu S, Sutter EE (1995) A topographic study of oscillatory potentials in man. Vis Neurosci 12(6):1013–1025
Vatcher D et al (2019) Revealing a retinal facilitatory effect with the multifocal ERG. Doc Ophthalmol 138(2):117–124
Zhou W et al (2007) Oscillatory potentials of the slow-sequence multifocal ERG in primates extracted using the Matching Pursuit method. Vis Res 47(15):2021–2036
Gauvin M et al (2015) Functional decomposition of the human ERG based on the discrete wavelet transform. J Vis 15(16):14
Gauvin M et al (2017) Quantifying the ON and OFF contributions to the flash ERG with the discrete wavelet transform. Transl Vis Sci Technol 6(1):3
Kurtenbach A, Jägle H (2008) Multifocal oscillatory potentials of the human retina. In: Tombran-Tink J (ed) Ophthalmology research. Humana Press, Totowa, pp 375–388
Song H et al (2011) Variation of cone photoreceptor packing density with retinal eccentricity and age. Invest Ophthalmol Vis Sci 52(10):7376–7384
Dolan RP, Schiller PH (1989) Evidence for only depolarizing rod bipolar cells in the primate retina. Vis Neurosci 2(5):421–424
Guite P, Lachapelle P (1990) The effect of 2-amino-4-phosphonobutyric acid on the oscillatory potentials of the electroretinogram. Doc Ophthalmol 75(2):125–133
Iwakabe H et al (1997) Impairment of pupillary responses and optokinetic nystagmus in the mGluR6-deficient mouse. Neuropharmacology 36(2):135–143
Masu M et al (1995) Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80(5):757–765
Schiller PH (1982) Central connections of the retinal ON and OFF pathways. Nature 297(5867):580–583
Slaughter MM, Miller RF (1981) 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211(4478):182–185
Takao M et al (2000) Impaired behavioral suppression by light in metabotropic glutamate receptor subtype 6-deficient mice. Neuroscience 97(4):779–787
Nelson R, Kolb H (2003) ON and OFF pathways in the vertebrate retina and visual system. In: Chalupa LM (ed) The visual neurosciences. MIT Press, Cambridge, pp 260–278
Wassle H et al (1989) Cortical magnification factor and the ganglion cell density of the primate retina. Nature 341(6243):643–646
Funding
Canadian Institutes of Health Research Grant # MOP126082.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors Allison L. Dorfman, Mathieu Gauvin, Dylan Vatcher, John M. Little, Robert C. Polomeno and Pierre Lachapelle declare that they have no conflict of interest to disclose.
Research involving human participants
This study was approved by the Institutional Ethics Review Board of the Montreal Children’s Hospital.
Statement on the welfare of animals
No animals were used in this study.
Informed consent
All procedures performed fulfilled the principles of the Declaration of Helsinki, and all participants signed an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
John M. Little died on June 10, 2018.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
Photopic ffERG (panel A) and mfERG (rings 4, 3, 2 and 1; panels B, C, D and E, respectively) waveforms obtained from all control subjects (blue tracings) and all cCSNB patients (red tracings). Major wave components are identified with corresponding letters (a, b, i and n-waves) or numbers (OP2, 3, 4). Response starts at time 0. Note in column A that the typical photopic ffERG waveform (i.e., square-wave-like a-wave, truncated b-wave, no oscillation on the ascending limb of the b-wave), which is pathognomonic for this condition, was seen in all our cCSNB patients (tracings 1 to 5). (JPEG 10811 kb)
Rights and permissions
About this article
Cite this article
Dorfman, A.L., Gauvin, M., Vatcher, D. et al. Ring analysis of multifocal oscillatory potentials (mfOPs) in cCSNB suggests near-normal ON–OFF pathways at the fovea only. Doc Ophthalmol 141, 99–109 (2020). https://doi.org/10.1007/s10633-020-09755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09755-2