Abstract
Purpose
The goal of the current study was to explore visual function in virally suppressed HIV patients undergoing combined antiretroviral therapy (cART) by using pattern-reversal and motion-onset visual evoked potentials (VEPs).
Methods
The pattern-reversal and motion-onset VEPs were recorded in 20 adult HIV+ patients with a mean age of 38 years and CD4 cell counts ≥230 × 106 cells/L of blood.
Results
Nine out of 20 patients displayed VEP abnormalities. Pattern-reversal VEPs pathology was observed in 20% of subjects, and 45% HIV patients had impaired motion-onset VEPs. Five out of 16 neurologically asymptomatic HIV patients had prolonged motion-onset VEP latencies in both eyes. Four neurologically symptomatic patients displayed simultaneously abnormal motion-onset and pattern-reversal VEP latencies: monocular involvement was observed in two patients with Lyme and cytomegalovirus unilateral optic neuritis. Binocular involvement was noted in two patients with cognitive deficits. Correlation analysis between disease duration, CD4 cell count, HIV copies in plasma, MoCA and electrophysiological parameters did not show any significant relationships.
Conclusions
The functional changes of the visual system in neurologically asymptomatic virally suppressed HIV patients displayed higher motion-onset VEP sensitivity than in standard pattern-reversal VEP examinations. This promising marker, however, has no significant association with clinical conditions. Further exploration is warranted.
Similar content being viewed by others
Introduction
Human immunodeficiency virus (HIV) is a member of the Lentivirus genus, part of the Retroviridae family and has neuroinvasive, neurotropic and neurovirulent properties [1, 2]. HIV infection leads to a wide range of clinical features because it may involve almost all systems including the nervous system and the optic nerve [3–5]. The examination of visual evoked potentials (VEPs) provides information about functional changes of the visual pathway to the brain visual cortex. Depending on the visual stimulation used, various subsystems of the visual pathway can be activated. The pattern reversal of a black/white checkerboard activates the primary visual area V1, which is reflected by a dominant positive P100 peak (P-VEPs) [6, 7]. The motion-onset visual evoked potentials (M-VEP) recordable via the activation of the magnocellular system and the dorsal stream of the visual pathway are characterized by the motion-onset-specific negative peak N160 in the extra-striatal temporooccipital and parietal cortices [8]. Axonal pathology in the optic nerve can be recognized by VEPs independently of overt clinical symptoms or detectable morphological changes [9, 10].
Before the introduction of combined antiretroviral therapy (cART), numerous studies reported neuro-ophthalmological and electrophysiological abnormalities, even in the early stages of infection. During the 1990s, prolonged latencies and reduced amplitudes of the P-VEPs and reduced amplitudes of the pattern electroretinogram (PERG) suggested retinal and postretinal visual pathway dysfunction in neurologically asymptomatic HIV-seropositive patients [11–17].
The advent of cART nearly two decades ago resulted in a profound decline in morbidity and mortality and raised the hope for an eradication of CNS complications [18]. The multifocal electroretinogram (mfERG) findings suggested early diffuse dysfunction of the inner retina resulted from severe HIV disease even in the cART era [19]. Surprisingly, relatively few P-VEP studies have reexamined the effect of HIV on CNS function since the widespread use of cART [20]. To our knowledge, no studies have used M-VEPs for the testing of visual motion processing in HIV-seropositive patients, neither before nor during the cART era. Therefore, the goal of the current study was to explore visual function in virally suppressed HIV patients undergoing cART by using pattern-reversal and motion-onset VEPs.
Methods
Patients
Between June 2013 and June 2015, 20 HIV+ patients who had visited the Department of Infectious Diseases (Faculty Hospital in Hradec Kralove in the Czech Republic) were enrolled into the electrophysiological study. Fourteen men (3 heterosexuals and 11 homosexuals) and 6 heterosexual women with serum antibodies to HIV as determined by ELISA and confirmed by Western blot were included. The demographic and clinical characteristics of the included patients are shown in Table 1. Their visual acuity is specified in Fig. 1. All patients had ≥230 × 106 CD4 cells/L of blood at the time of enrollment. The duration of HIV infection was estimated considering the first positive HIV antibody test. It was found to be 0.1–20.7 years (median 3.5 years) at the time of inclusion in the study. The mean age of patients at inclusion was 38 years (2 patients were ≤25 years and 3 subjects were >45 years). The HIV disease staging classification according to the Centers for Disease Control and Prevention (CDC) was determined in all patients (A1–C3) [21]. The CDC categorization of HIV/AIDS is based on the lowest documented CD4 cell count as well as previously diagnosed HIV-related conditions. The International AIDS Society—USA Panel advises the start of treatment in patients with symptomatic HIV disease and patients with CD4 cell counts below 350 × 106 CD4 cells/L of blood or viral load above 50,000–100,000 copies/mL of blood [22]. At inclusion, 6 patients had not undergone any treatment for HIV. Fourteen HIV patients had been taking antiretroviral medication for at least 2 months before participation in the study (median 4.4 years). All of these patients had a combination of different groups of antiretroviral medicines into one complete regimen (protease inhibitor + ritonavir, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors or integrase inhibitor). The Montreal Cognitive Assessment (MoCA) was used as a screening instrument for a cognitive function evaluation. A score of ≤25 was considered to represent cognitive impairment [23].
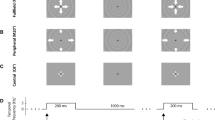
Monocular pattern-reversal VEPs (40′ and 20′) are plotted in four columns. The subject responses are organized in rows and sorted by age so that the eldest subject is in the last row. The subject numbers correspond to those of Table 1. The green lines indicate the upper borders (M + 2.5 SD) of the latency norm (Table 2) with respect to the age of each subject. Pathological responses are plotted by red lines. Corresponding visual acuity obtained by the Landolt C test is listed in the right-most columns of the figure. Abbreviations are consistent with those of Table 2
Visual evoked potentials (VEPs)
The VEPs were examined in the Electrophysiological laboratory at Charles University in Prague—Faculty of Medicine in Hradec Kralove. Two types of visual stimuli were used to elicit VEPs (pattern-reversal and motion-onset stimuli) with the aim of testing the function of the two fundamental subsystems of the visual pathway and their cortical projections.
In the pattern-reversal stimulation (P-VEP), a black and white checkerboard (contrast 96% according to Michelson) with check sizes of 40′ and 20′ was reversed at a frequency of 2/s [24].
Motion-onset stimuli (M-VEP) consisted of low contrast (10%) gray concentric circles that randomly expanded/contracted (radial motion). We recorded three variants of M-VEP responses to the onset of radial motion: (1) a stimulus comprising 37° × 28° of the central visual field (FF); (2) a stimulus field outside the central 20° (M20°); and (3) the central 8° of the visual field (C8°). All motion-onset stimuli had the same timing: 200 ms of motion followed by a 1-second interstimulus interval (stationary pattern) [8, 25].
The stimuli were presented on a 21” computer monitor (Vision Master Pro 510, Iiyama, Japan) subtending 37° × 28° of the visual field at a 0.6 m viewing distance. The monitor was driven using a Visual Stimulus Generator 2/5 (CRS Ltd., UK) at a 105-Hz vertical refresh frequency. A mean luminance of 17 cd/m2 was used for all stimuli. The correct fixation of the stimulus field center was monitored via a near-infrared camera. Forty single sweeps were averaged because this number of sweeps in our recording conditions with the Faraday cage provides a good signal-to-noise ratio. Monocular VEPs were recorded from six unipolar derivations using the right ear lobe as a reference. Four derivations from the midline (Oz, Pz, Cz and Fz) and two lateral derivations (Ol and Or that were 5 cm to the left and right from the Oz position, respectively) were used to cover areas with maximum amplitudes for both the P-VEPs and M-VEPs. Latencies and interpeak amplitudes of the P100 peak were always evaluated in the Oz derivation. Because of interindividual differences in the topographies of the M-VEPs [26], latencies and interpeak amplitudes of the N160 motion-onset-specific peak were read from one of the lateral occipital or Pz leads. We selected the lead with the maximum interpeak amplitude.
Individual VEP parameters were compared to laboratory normal values. Norms were derived from a control group of 70 healthy subjects with significant age-dependent VEPs changes [27, 28]. VEP latencies that exceeded the value of the mean + 2.5 standard deviation were considered as pathologically prolonged. The upper limits of the latency norms are shown in Table 2.
Interocular latency differences over 10 ms in the P100 peak and 20 ms in the N160 peak were also recognized as pathological.
Systematic interocular differences of the VEP amplitudes exceeding approximately 3 µV appeared to be significant for the detection of unilateral pathology. VEP amplitudes in single eyes were not used for pathological detection due to known significant interindividual variability [29].
Statistical analyses
The data were statistically processed with R software version 3.2 [30], using the “nortest”, “psych” and “ggplot2” packages. Because the Anderson–Darling test had rejected the hypothesis of normal data distribution, the Spearman nonparametric test of the relationship between clinical and electrophysiological parameters was used. A relationship was considered to be statistically significant when the probability level (P) was below an alpha level of 0.05. The alpha level was corrected for multiple comparisons by a Holm–Bonferroni adjustment. The Kruskal–Wallis nonparametric test was used for assessment of differences among three groups defined by the clinical severity scale (CDC) in pursued electrophysiological or clinical parameters.
Results
The 20 cooperating patients were divided into two groups: (1) neurologically symptomatic subjects (4/20) and (2) neurologically asymptomatic patients (16/20).
Neurologically symptomatic subjects with cognitive impairment—2 cases
A heterosexual couple with no visual or other neurological complaints and an unclear duration of infection before the incidental detection of HIV scored in the MoCA 19/30 points (female) and 22/30 points (male), below the normal limit (26/30 points). Woman No. 19 displayed prolonged M-VEP latencies in both eyes and normal P-VEP reactions. Man No. 20 had prolonged R20′ P-VEP latencies in both eyes and a M20° M-VEP latency delay in one eye (for details, see Figs. 1, 2).
Neurologically symptomatic subjects with neuro-ophthalmological manifestation—2 cases
Patient No. 1, who was adherent to the cART therapy inconsistently, complained of a few weeks of blurred vision in the right eye. VEP examination showed absent reactions to P-VEP and small M-VEP reactions with prolonged latencies in the right eye (M-VEP latencies: FF 234 ms, M20° 244 ms, C8° no reaction; interpeak amplitudes: FF 4.5 µV, M20° 5.1 µV, C8° 3.5 µV). The left eye showed completely normal reactions for left eye stimulation (P-VEP latencies R40′ 106 ms, R20′ 118 ms; interpeak amplitudes: R40′ 14.8 µV, R20′ 17.1 µV and M-VEP latencies FF 140 ms, M20° 144 ms, C8° 158 ms; interpeak amplitudes: FF 16.1 µV, M20° 13 µV, C8° 6.3 µV). These findings, together with clinical symptoms, suggested the presence of acute optic neuritis. The brain magnetic resonance imaging suspicion of cytomegalovirus (CMV) encephalopathy with right eye retinitis and facial nerve palsy on the right side was confirmed by positive polymerase chain reaction in the cerebrospinal fluid (CSF-PCR) for CMV-DNA.
Patient No. 15 suffered from a headache, diffuse arthralgia and fever. Lyme borreliosis coinfection was detected with a high titer IgG in the blood (ELISA, TestLine, Brno, Czech Republic) and symptoms resolving within 14 days after oral doxycycline therapy. Three months later, the patient without any visual difficulties had an electrophysiological picture of monocular optic nerve involvement with prolonged latencies in the P-VEP and M-VEPs of the right eye with a VEP recovery to normal within 1 year.
Neurologically asymptomatic patients—16 cases
Eleven patients with duration of HIV infection between 0.1 and 20.7 years had VEP reactions within our laboratory limits.
In 5 cases, the VEP evaluation revealed a binocular pathology. Patients with an HIV infection for 2.9–8 years without visual or neurological difficulties displayed prolonged latencies or slurred reactions in M-VEPs in all motion variants. Abnormal M-VEPs were accompanied by P-VEP latency delay in only case No. 17.
Effect of clinical severity (CDC score)
Three subgroups divided by the CDC clinical criteria were defined as follows: group a: A1 + A2 + A3, 10 patients, group b: B1 + B2 + B3, 6 patients, and group c: C1 + C2 + C3, 4 patients. The Kruskal–Wallis nonparametric ANOVA did not show any significant differences among the sensory parameters, the cognitive test and the biochemical markers—see Fig. 3.
The boxplots illustrate medians, interquartile ranges, minimum and maximum CD4 cell counts (top) and HIV copies in plasma (bottom) among three groups defined by CDC clinical score (group a = A1 + A2 + A3, 10 patients, group b = B1 + B2 + B3, 6 patients, and group c = C1 + C2 + C3, 4 patients). The nonparametric Kruskal–Wallis test did not show any intergroup differences as well as differences among other followed parameters (not depicted here)
Relationship among sensory/cognitive and clinical parameters
The Spearman rank correlation test of relationships between disease duration, CD4, HIV copies in plasma, MoCA and electrophysiological parameters also did not show any significant results. The independence between VEPs latencies of R40′ or expanding FF and CD4 cell counts at the time of inclusion in the study is demonstrated in Fig. 4. The graphs show that visual pathology was not related to the CD4 cell count, which is coded as the size of dots.
A weak relationship between visual pathology and the CD4 cell count is illustrated by the P100 latency of the pattern-reversal R40′ VEP in the upper plot and the N160 latency of the motion-onset FF VEP in the bottom plot. Every point position represents a subject’s left and right eye response, and the size of the point corresponds to the CD4 cell count. Normal VEP findings are located in gray areas, and normal CD4 counts are green colored
Discussion
Before the widespread use of cART, electrophysiological studies reported optic nerve dysfunction in 1–45% of neurologically asymptomatic HIV-seropositive patients as detected by P-VEPs [11–16].
In the present study, P-VEP pathology was observed in 3 neurologically symptomatic subjects, specifically in two with Lyme and cytomegalovirus infection, as well as in one patient with cognitive impairment. Only 1 of 16 neurologically asymptomatic patients with bilaterally prolonged M-VEP latencies in all motion variants showed concurrent P-VEP R40′ latency delay in one eye.
The P-VEP is regarded as a sensitive marker for the detection of optic neuritis. In HIV+ patients, most cases of optic neuritis are associated with opportunistic infections [3]. However, the advent of cART has reduced the incidence and severity of opportunistic infections in the HIV-infected population [31]. Our study supports the proposition that the initiation of cART therapy for HIV patients has substantially reduced the risk of impaired optic nerve function due to opportunistic infections. In the present study, not a single patient in our group treated with cART had clinical signs or VEP evidence of acute optic neuritis or residual effects after the neuritis except for one patient with Lyme borreliosis coinfection who had a VEP picture of monocular optic nerve involvement with recovery within 1 year after antibiotic treatment. The only patient refusing cART treatment with 280 × 106 CD4 cells/L and a viral load of 310,000 copies/mL suffered from optic neuritis as a manifestation of opportunistic infections. The VEP picture of optic neuritis in our study is characterized by the monocular influence of the P-VEP with the finding of the current involvement of M-VEP. Such a VEP image is also found in patients with optic neuritis in multiple sclerosis or neuroborreliosis [32, 33].
In our study, 4 out of 16 neurologically asymptomatic virally suppressed HIV patients had a prolongation of the motion-onset-specific N160 peak in both eyes with normal P-VEP findings. In 3 out of these 4 patients, the ERG, PERG, mfERG and funduscopic examination was additionally performed and no evidence of the retina damage was found. The fourth patient with the same VEP results was not examined because he committed suicide in the meantime.
Although VEP examination is not specific enough to localize impairment, we may speculate that the likelihood of cortical involvement is greater than that of an optic nerve lesion because the latter is unlikely to cause binocular M-VEP pathology and spared ERGs and P-VEPs as we observed in our patients. Moreover, the N160 peak is generated in the extra-striatal temporooccipital and parietal cortices [34], and a loss of cortical neurons in these regions has been found in asymptomatic HIV-positive subjects in the early stages of infection [35] and in many neuroimaging studies [36–40]. Therefore, we hypothesize that motion-onset VEPs may help to detect early central nervous system involvement in HIV-positive subjects.
The viewpoint of broader cortical impairment is further underlined by two patients with cognitive deficits and pathological VEPs. However, we cannot speculate about the role of HIV infection in their cognitive deficits because their pre-disease states were unknown.
Only one patient suffering from CMV encephalopathy with optic neuritis had a sudden decrease in vision in one eye. No visual complaints in 19 of twenty patients indicate that despite normal visual acuity, the subclinical dysfunction of the visual system may be present in HIV+ patients. In such a situation, VEPs could serve as a sensitive insight into disease development as they have been demonstrated in optic nerve pathology in patients without overt clinical symptoms [10, 12, 41].
For a deeper understanding of the functional changes in the visual system and CNS in HIV patients on cART, we plan to observe the long-term development of VEP findings in a follow-up study.
Conclusion
In the current study, nine of 20 HIV patients displayed electrophysiological abnormalities. We found abnormal P-VEP in 20% of HIV patients and M-VEP pathology in 45% of HIV+ subjects. Impairment of the P-VEP was predominantly observed in neurologically symptomatic subjects.
Prolongation of the M-VEP was detected in patients with neuro-ophthalmological symptoms as well as in neurologically asymptomatic subjects. The functional changes of the visual system detectable with the use of motion-onset VEPs appeared separately from the changes detected by the standard pattern-reversal VEP examination. The similar results of higher motion-onset VEP sensitivity in various CNS pathologies have been previously reported by our laboratory [8, 42, 43].
Limitations
The small group of the patients examined and the wide range of the duration of HIV infection (low homogeneity of the group) certainly represent shortcomings of this study. Unfortunately, the real duration of infection was known for only a small number of patients. Furthermore, the duration of infection was derived from the first positive HIV antibody test in most subjects.
Another shortcoming of the study is the lack of information about the functional changes in the retina and intraorbital portion of the optic nerve in most patients. Optic nerve fundus investigation was not included in this study.
References
Patrick MK, Johnston JB, Power C (2002) Lentiviral neuropathogenesis: comparative neuroinvasion, neurotropism, neurovirulence, and host neurosusceptibility. J Virol 76:7923–7931
Chiodi F, Fenyo EM (1991) Neurotropism of human immunodeficiency virus. Brain Pathol 1:185–191
Mwanza JC, Nyamabo LK, Tylleskar T, Plant GT (2004) Neuro-ophthalmological disorders in HIV infected subjects with neurological manifestations. Br J Ophthalmol 88:1455–1459
Jabs DA (1995) Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 93:623–683
Boisse L, Gill MJ, Power C (2008) HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin 26:799–819
Di Russo F, Pitzalis S, Spitoni G, Aprile T, Patria F, Spinelli D, Hillyard SA (2005) Identification of the neural sources of the pattern-reversal VEP. Neuroimage 24:874–886
Barnikol UB, Amunts K, Dammers J, Mohlberg H, Fieseler T, Malikovic A, Zilles K, Niedeggen M, Tass PA (2006) Pattern reversal visual evoked responses of V1/V2 and V5/MT as revealed by MEG combined with probabilistic cytoarchitectonic maps. Neuroimage 31:86–108
Kuba M, Kubova Z, Kremlacek J, Langrova J (2007) Motion-onset VEPs: characteristics, methods, and diagnostic use. Vis Res 47:189–202
Sadun AA, Pepose JS, Madigan MC, Laycock KA, Tenhula WN, Freeman WR (1995) AIDS-related optic neuropathy: a histological, virological and ultrastructural study. Graefes Arch Clin Exp Ophthalmol 233:387–398
Mahadevan A, Satishchandra P, Prachet KK, Sidappa NB, Ranga U, Santosh V, Yasha TC, Desai A, Ravi V, Shankar SK (2006) Optic nerve axonal pathology is related to abnormal visual evoked responses in AIDS. Acta Neuropathol 112:461–469
Malessa R, Heuser-Link M, Brockmeyer N, Goos M, Schwendemann G (1989) Evoked potentials in neurologically asymptomatic persons during the early stages of HIV infection. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb 20:257–266
Malessa R, Agelink MW, Diener HC (1995) Dysfunction of visual pathways in HIV-1 infection. J Neurol Sci 130:82–87
Pierelli F, Soldati G, Zambardi P, Garrubba C, Spadaro M, Tilia G, Pauri F, Morocutti C (1993) Electrophysiological study (VEP, BAEP) in HIV-1 seropositive patients with and without AIDS. Acta Neurol Belg 93:78–87
Somma-Mauvais H, Farnarier G (1992) Evoked potentials in HIV infection. Neurophysiol Clin 22:369–384
Iragui VJ, Kalmijn J, Plummer DJ, Sample PA, Trick GL, Freeman WR (1996) Pattern electroretinograms and visual evoked potentials in HIV infection: evidence of asymptomatic retinal and postretinal impairment in the absence of infectious retinopathy. Neurology 47:1452–1456
Farnarier G, Somma-Mauvais H (1990) Multimodal evoked potentials in HIV infected patients. Electroencephalogr Clin Neurophysiol Suppl 41:355–369
Plummer DJ, Sample PA, Freeman WR (1998) Visual dysfunction in HIV-positive patients without infectious retinopathy. Aids Patient Care STDS 12:171–179
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860
Falkenstein IA, Bartsch DU, Azen SP, Dustin L, Sadun AA, Freeman WR (2008) Multifocal electroretinography in HIV-positive patients without infectious retinitis. Am J Ophthalmol 146:579–588
Goldsmith P, Jones RE, Ozuzu GE, Richardson J, Ong EL (2000) Optic neuropathy as the presenting feature of HIV infection: recovery of vision with highly active antiretroviral therapy. Br J Ophthalmol 84:551–553
Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL (1993) revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 41:1–19
Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Volberding PA (2002) Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 288:222–235
Valcour VG (2011) Evaluating cognitive impairment in the clinical setting: practical screening and assessment tools. Top Antivir Med 19:175–180
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, Vaegan (2010) ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 120:111–119
Kremlacek J, Kuba M, Kubova Z, Chlubnova J (2004) Motion-onset VEPs to translating, radial, rotating and spiral stimuli. Doc Ophthalmol 109:169–175
Kuba M, Kubova Z (1992) Visual evoked potentials specific for motion onset. Doc Ophthalmol 80:83–89
Langrova J, Kuba M, Kremlacek J, Kubova Z, Vit F (2006) Motion-onset VEPs reflect long maturation and early aging of visual motion-processing system. Vis Res 46:536–544
Kuba M, Kremlacek J, Langrova J, Kubova Z, Szanyi J, Vit F (2012) Aging effect in pattern, motion and cognitive visual evoked potentials. Vis Res 62:9–16
Snyder EW, Dustman RE, Shearer DE (1981) Pattern reversal evoked potential amplitudes: life span changes. Electroencephalogr Clin Neurophysiol 52:429–434
R Core Team (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. Accessed 19 May 2016
Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H (2009) Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58:1–198
Szanyi J, Kuba M, Kubova Z, Kremlacek J, Langrova J, Jiraskova N (2008) Retrospective analysis of visual evoked potentials findings in acute retrobulbar neuritis. Cesk Slov Neurol 71:317–323
Szanyi J, Kubova Z, Kremlacek J, Langrova J, Vit F, Kuba M, Szanyi J, Plisek S (2012) Pattern and motion-related visual-evoked potentials in neuroborreliosis: follow-up study. J Clin Neurophysiol 29:174–180
Pitzalis S, Strappini F, De Gasperis M, Bultrini A, Di Russo F (2012) Spatio-temporal brain mapping of motion-onset VEPs combined with fMRI and retinotopic maps. PLoS ONE 7:e35771
Tran Dinh YR, Mamo H, Cervoni J, Caulin C, Saimot AC (1990) Disturbances in the cerebral perfusion of human immune deficiency virus-1 seropositive asymptomatic subjects: a quantitative tomography study of 18 cases. J Nucl Med 31:1601–1607
Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci USA 102:15647–15652
Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M (2011) Structural gray and white matter changes in patients with HIV. J Neurol 258:1066–1075
Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW (2009) Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol 15:324–333
Li Y, Li H, Gao Q, Yuan D, Zhao J (2014) Structural gray matter change early in male patients with HIV. Int J Clin Exp Med 7:3362–3369
Samuelsson K, Pirskanen-Matell R, Bremmer S, Hindmarsh T, Nilsson BY, Persson HE (2006) The nervous system in early HIV infection: a prospective study through 7 years. Eur J Neurol 13:283–291
Moodley A, Rae W, Bhigjee A, Connolly C, Devparsad N, Michowicz A, Harrison T, Loyse A (2012) Early clinical and subclinical visual evoked potential and Humphrey’s visual field defects in cryptococcal meningitis. PLoS ONE 7:e52895
Kubova Z, Szanyi J, Langrova J, Kremlacek J, Kuba M, Honegr K (2006) Motion-onset and pattern-reversal visual evoked potentials in diagnostics of neuroborreliosis. J Clin Neurophysiol 23:416–420
Kremlacek J, Valis M, Masopust J, Urban A, Zumrova A, Talab R, Kuba M, Kubova Z, Langrova J (2011) An electrophysiological study of visual processing in spinocerebellar ataxia type 2 (SCA2). Cerebellum 10:32–42
Acknowledgements
This work was supported by Charles University in Prague, Czech Republic, Project PRVOUK P37/07.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interests for any of the authors. The authors have nothing to disclose. J.S., J.K., Z.K., M.K., P.G., F.V. and J. L. were supported by a grant from Charles University in Prague, Czech Republic—PRVOUK P37/07.
Ethical approval
The study was approved by the Ethical Committee of the University Hospital in Hradec Kralove. All experiments were conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent to our VEP examination was obtained from each subject.
Rights and permissions
About this article
Cite this article
Szanyi, J., Kremlacek, J., Kubova, Z. et al. Pattern- and motion-related visual evoked potentials in HIV-infected adults. Doc Ophthalmol 134, 45–55 (2017). https://doi.org/10.1007/s10633-016-9570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-016-9570-x