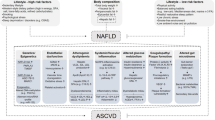
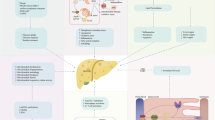
Abstract
Background
Measures of insulin resistance (IR)/sensitivity (IS) are emerging tools to identify metabolic-associated fatty liver disease (MAFLD). However, the comprehensive assessment of the performance of various indicators is limited. Moreover, the utility of measures of IR/IS in detecting liver fibrosis remains unclear.
Aims
To evaluate the predictive ability of seventeen IR/IS and two beta cell function indices to identify MAFLD and liver fibrosis.
Methods
A cross-sectional study was conducted on individuals aged 25–75 years. Transient elastography was used to estimate liver stiffness and controlled attenuation parameter. The following measures were computed: homeostatic model assessment (HOMA/HOMA2) for IR, IS, and beta cell function; QUICKI; Bennett index; glucose/insulin; FIRI; McAuley index; Reynaud index; SPISE index; TyG; TyG-BMI; TyG-WC; TyG-WHtR; TG/HDL; and METS-IR. Subgroup analyses were performed according to age, gender, diabetes status, and body weight.
Results
A total of 644 individuals were included in our analysis. MAFLD and significant liver fibrosis were detected in 320 (49.7%) and 80 (12.4%) of the participants, respectively. All measures of IR/IS identified MAFLD and liver fibrosis. However, TyG-WC, TyG-BMI, and TyG-WHtR were the top three indicators that identified MAFLD. Measures that include insulin level in their mathematical calculation, namely, Raynaud index, HOMA-IR, HOMA 2-IR, FIRI, and QUICKI had the best performance in identifying liver fibrosis in the entire population, as well as among the study subgroups.
Conclusions
TyG-WC, TyG-BMI, and TyG-WHtR were the best predictors of MAFLD. Insulin-based measures had better performances in the detection of advanced fibrosis. This was independent of age, gender, obesity, or diabetes status.


Similar content being viewed by others
References
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. https://doi.org/10.1038/nrgastro.2017.109.
Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: what we need in the future. Liver Int. 2018;38:47–51. https://doi.org/10.1111/liv.13643.
Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3:100305. https://doi.org/10.1016/j.jhepr.2021.100305.
Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017. https://doi.org/10.3390/nu9040387.
Koo DJ, Lee MY, Jung I, Moon SJ, Kwon H, Park SE et al. Baseline homeostasis model assessment of insulin resistance associated with fibrosis progression in patients with nonalcoholic fatty liver disease without diabetes: a cohort study. PLoS One. 2021;16:e0255535. https://doi.org/10.1371/journal.pone.0255535.
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–944. https://doi.org/10.1111/jgh.13264.
Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999-2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312.
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082–2089. https://doi.org/10.1111/liv.14548.
Alba LM, Lindor K. Review article: non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2003;17:977–986. https://doi.org/10.1046/j.1365-2036.2003.01493.x.
Singh SP, Barik RK. NonInvasive biomarkers in nonalcoholic fatty liver disease: are we there yet? J Clin Exp Hepatol. 2020;10:88–98. https://doi.org/10.1016/j.jceh.2019.09.006.
Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M et al. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, Placebo-controlled trial. Adv Ther. 2020;37:4697–4708. https://doi.org/10.1007/s12325-020-01498-5.
Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, Malek M, Reza Babaei M, Zamani F et al. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Diabet Ther. 2021;12:843–861. https://doi.org/10.1007/s13300-021-01011-3.
Poustchi H, Alaei-Shahmiri F, Aghili R, Nobarani S, Malek M, Khamseh ME. Hepatic steatosis and fibrosis in type 2 diabetes: a risk-based approach to targeted screening. Arch Iran Med. 2021;24:177–186. https://doi.org/10.34172/aim.2021.28.
Salavatizadeh M, Soltanieh S, Poustchi H, Yari Z, Shabanpur M, Mansour A et al. Dietary total antioxidant capacity is inversely associated with the odds of non-alcoholic fatty liver disease in people with type-2 diabetes. Front Nutr. 2022;9:1037851. https://doi.org/10.3389/fnut.2022.1037851.
Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. https://doi.org/10.1016/s2468-1253(19)30383-8.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. https://doi.org/10.1053/j.gastro.2019.01.042.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. https://doi.org/10.1016/j.jhep.2020.03.039.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. https://doi.org/10.1007/bf00280883.
The Oxford Centre for Diabetes. Endocrinology & metabolism. Diabetes trial unit. HOMA calculator. http://www.dtu.ox.ac.uk.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabet Care. 1998;21:2191–2192. https://doi.org/10.2337/diacare.21.12.2191%JDiabetesCare.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. https://doi.org/10.1210/jcem.85.7.6661.
Anderson RL, Hamman RF, Savage PJ, Saad MF, Laws A, Kades WW et al. Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The insulin resistance atherosclerosis study. Am J Epidemiol. 1995;142:724–732. https://doi.org/10.1093/aje/142.7.724.
Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome1. J Clin Endocrinol Metab. 1998;83:2694–2698. https://doi.org/10.1210/jcem.83.8.5054.
Duncan MH, Singh BM, Wise PH, Carter G, Alaghband-Zadeh J. A simple measure of insulin resistance. Lancet. 1995;346:120–121. https://doi.org/10.1016/S0140-6736(95)92143-5.
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA et al. Diagnosing insulin resistance in the general population. Diabet Care. 2001;24:460–464. https://doi.org/10.2337/diacare.24.3.460.
Raynaud E, Perez-Martin A, Brun JF, Benhaddad AA, Mercier J. Revised concept for the estimation of insulin sensitivity from a single sample. Diabet Care. 1999;22:1003–1004. https://doi.org/10.2337/diacare.22.6.1003.
Paulmichl K, Hatunic M, Højlund K, Jotic A, Krebs M, Mitrakou A et al. Modification and validation of the triglyceride-to-HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the single point insulin sensitivity estimator (SPISE). Clin Chem. 2016;62:1211–1219. https://doi.org/10.1373/clinchem.2016.257436.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, MaG Ramos-Zavala, Hernández-González SO et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. https://doi.org/10.1210/jc.2010-0288.
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11:e0149731. https://doi.org/10.1371/journal.pone.0149731.
Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14:260. https://doi.org/10.1186/s12967-016-1020-8.
Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963. https://doi.org/10.1371/journal.pone.0212963.
Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS One. 2016;11:e0154345. https://doi.org/10.1371/journal.pone.0154345.
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Annals Intern Med. 2003;139:802–809. https://doi.org/10.7326/0003-4819-139-10-200311180-00007.
Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533–544. https://doi.org/10.1530/eje-17-0883.
Beran A, Ayesh H, Mhanna M, Wahood W, Ghazaleh S, Abuhelwa Z et al. Triglyceride-glucose index for early prediction of nonalcoholic fatty liver disease: a meta-analysis of 121,975 individuals. J Clin Med. 2022. https://doi.org/10.3390/jcm11092666.
Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The Diagnostic and prognostic value of the triglyceride-glucose index in metabolic dysfunction-associated fatty liver disease (MAFLD): a systematic review and meta-analysis. Nutrients. 2022;14:4969.
Taheri E, Pourhoseingholi MA, Moslem A, Hassani AH, Mousavi Jarrahi A, Asadzadeh Aghdaei H et al. The triglyceride-glucose index as a clinical useful marker for metabolic associated fatty liver disease (MAFLD): a population-based study among Iranian adults. J Diabet Metab Disord. 2022;21:97–107. https://doi.org/10.1007/s40200-021-00941-w.
Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19:167–173. https://doi.org/10.1089/met.2020.0109.
Wang R, Dai L, Zhong Y, Xie G. Usefulness of the triglyceride glucose-body mass index in evaluating nonalcoholic fatty liver disease: insights from a general population. Lipids Dis. 2021;20:77. https://doi.org/10.1186/s12944-021-01506-9.
Song S, Son DH, Baik SJ, Cho WJ, Lee YJ. Triglyceride glucose-waist circumference (TyG-WC) is a reliable marker to predict non-alcoholic fatty liver disease. Biomedicines. 2022. https://doi.org/10.3390/biomedicines10092251.
Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol. 2022;13:951689. https://doi.org/10.3389/fendo.2022.951689.
Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. https://doi.org/10.1016/j.metabol.2016.02.014.
Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15:21–35. https://doi.org/10.1007/s12072-020-10121-2.
Bae JC, Beste LA, Utzschneider KM. The impact of insulin resistance on hepatic fibrosis among United States adults with non-alcoholic fatty liver disease: NHANES 2017 to 2018. Endocrinol Metab (Seoul). 2022;37:455–465. https://doi.org/10.3803/EnM.2022.1434.
Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. https://doi.org/10.1186/s12944-017-0572-9.
Fujii H, Imajo K, Yoneda M, Nakahara T, Hyogo H, Takahashi H et al. HOMA-IR: An independent predictor of advanced liver fibrosis in nondiabetic non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34:1390–1395. https://doi.org/10.1111/jgh.14595.
Kim MK, Kim JH, Park K, Lee SB, Nam JS, Kang S et al. Relationship between the triglyceride glucose index and the presence and fibrosis of nonalcoholic fatty liver disease in Korean adults. Am Diabet Assoc. 2018. https://doi.org/10.1530/endoabs.56.P296.
Koo DJ, Lee MY, Jung I, Moon SJ, Kwon H, Park SE et al. Changes in insulin resistance index and the risk of liver fibrosis in patients with nonalcoholic fatty liver disease without diabetes: Kangbuk Samsung Health Study. Endocrinol Metab. 2021;36:1016–1028. https://doi.org/10.3803/EnM.2021.1110.
Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:2178–2187. https://doi.org/10.1002/hep.26988.
Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabet. 2008;9:57–61.
Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. https://doi.org/10.1186/1471-2288-11-158.
Kato K, Takeshita Y, Misu H, Zen Y, Kaneko S, Takamura T. Liver steatosis is associated with insulin resistance in skeletal muscle rather than in the liver in Japanese patients with non-alcoholic fatty liver disease. J Diabet Investig. 2015;6:158–163. https://doi.org/10.1111/jdi.12271.
Lee WH, Najjar SM, Kahn CR, Hinds TD Jr. Hepatic insulin receptor: new views on the mechanisms of liver disease. Metabolism. 2023;145:155607. https://doi.org/10.1016/j.metabol.2023.155607.
Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. https://doi.org/10.1002/hep.510290632.
Cai CX, Buddha H, Castelino-Prabhu S, Zhang Z, Britton RS, Bacon BR et al. Activation of insulin-PI3K/Akt-p70S6K pathway in hepatic stellate cells contributes to fibrosis in nonalcoholic steatohepatitis. Dig Dis Sci. 2017;62:968–978. https://doi.org/10.1007/s10620-017-4470-9.
Masuda K, Noguchi S, Ono M, Ochi T, Munekage K, Okamoto N et al. High fasting insulin concentrations may be a pivotal predictor for the severity of hepatic fibrosis beyond the glycemic status in non-alcoholic fatty liver disease patients before development of diabetes mellitus. Hepatol Res. 2017;47:983–990. https://doi.org/10.1111/hepr.12832.
Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. https://doi.org/10.1053/jhep.2001.28055.
Zhang F, Zhang Z, Kong D, Zhang X, Chen L, Zhu X et al. Tetramethylpyrazine reduces glucose and insulin-induced activation of hepatic stellate cells by inhibiting insulin receptor-mediated PI3K/AKT and ERK pathways. Mol Cell Endocrinol. 2014;382:197–204. https://doi.org/10.1016/j.mce.2013.09.020.
Chen X, Xiao J, Pang J, Chen S, Wang Q, Ling W. Pancreatic β-cell dysfunction is associated with nonalcoholic fatty liver disease. Nutrients. 2021. https://doi.org/10.3390/nu13093139.
Siddiqui MS, Cheang KL, Luketic VA, Boyett S, Idowu MO, Patidar K et al. Nonalcoholic steatohepatitis (NASH) is associated with a decline in pancreatic beta cell (β-cell) function. Dig Dis Sci. 2015;60:2529–2537. https://doi.org/10.1007/s10620-015-3627-7.
Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R. Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology. 2012;56:933–942. https://doi.org/10.1002/hep.25739.
Araki N, Takahashi H, Takamori A, Kitajima Y, Hyogo H, Sumida Y et al. Decrease in fasting insulin secretory function correlates with significant liver fibrosis in Japanese non-alcoholic fatty liver disease patients. JGH Open. 2020;4:929–936. https://doi.org/10.1002/jgh3.12367.
Al-Mrabeh A. β-cell dysfunction, hepatic lipid metabolism, and cardiovascular health in type 2 diabetes: new directions of research and novel therapeutic strategies. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9020226.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-64. https://doi.org/10.1016/j.jhep.2014.12.012.
Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019;40:1367–1393. https://doi.org/10.1210/er.2019-00034.
Trowell J, Alukal J, Zhang T, Liu L, Maheshwari A, Yoo HY et al. How good are controlled attenuation parameter scores from fibroscan to assess steatosis, NASH, and fibrosis? Dig Dis Sci. 2021;66:1297–1305. https://doi.org/10.1007/s10620-020-06269-4.
Acknowledgments
This study was supported by the Iran University of Medical Sciences (IUMS) (Research project number: 1401-3-116-24435).
Author information
Authors and Affiliations
Contributions
FIB, MEK, MM, and FASh were involved in the conception and design of the study. Data were collected by SN, AH, MS, SS, HC, HT, ZM, and FA. SJ and FASh were involved in drafting of the manuscript. All authors have participated in reviewing the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The current study was carried out under the Helsinki Declaration and approved by the ethics committee of the Iran University of Medical Sciences (Approval code: IR.IUMS.REC.1401.821).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khamseh, M.E., Malek, M., Jahangiri, S. et al. Insulin Resistance/Sensitivity Measures as Screening Indicators of Metabolic-Associated Fatty Liver Disease and Liver Fibrosis. Dig Dis Sci 69, 1430–1443 (2024). https://doi.org/10.1007/s10620-024-08309-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08309-9