Abstract
Background
Patients with inflammatory bowel disease (IBD) are at increased risk for many co-morbid diseases. However, little is known about durability of IBD medications in patients with co-morbid diseases.
Aims
Determine medication durability in IBD patients with and without psoriasis, rheumatoid arthritis, and/or enteropathic arthropathy.
Methods
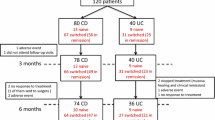
All patients with at least three ICD-9 or 10 diagnoses for IBD were included in the cohort. The risk factors of interest were a co-morbid diagnosis of psoriasis (IBD-Ps), rheumatoid arthritis (IBD-RA), and/or enteropathic arthritis (IBD-EA). Medication durability was defined as days of medication use, calculated using order start and stop dates from the electronic medical record. Significant differences were tested using the Wilcoxon rank sum test for continuous variables and Fisher’s exact test or Pearson’s Chi-squared test, as appropriate, for categorical variables. Boxplots were constructed for graphical interpretation of results.
Results
In the psoriasis group, there were 481 patients with 831 medication exposures [131 IBS-Ps (16%), 700 IBD only (84%)]. The median days of medication use were numerically higher in the IBD-Ps group for all therapies [anti-TNF: 1109 vs 861 (p = 0.17); anti-IL-12/23: 984 vs 834 (p = 0.33); JAKi: 682 vs 230 (p = 0.13)], anti-TNF/IM: 370 vs 202 (p = 0.57), except anti-integrin therapy [214 vs 470 (p = 0.08)]. When restricting to UC only, patients with co-morbid again Ps had a significantly shorter duration on anti-integrin therapy (84 vs 456 days, p = 0.02). While not reaching statistical significance, there was a distinctly longer medication duration on JAKi therapy (910 vs 317, p = 0.10). When restricting to patients with CD only, no results reached statistical significance though there was a trend towards longer anti-TNF durability in CD-Ps (1340 vs 1000 days, p = 0.098). There were no differences in medication durability in IBD-RA or IBD-EA patients.
Discussion
Larger studies investigating medication durability of JAKi and anti-integrin therapy in IBD patients with psoriasis would be beneficial given noteworthy trends towards increased and decreased durability, respectively.




Similar content being viewed by others
References
Bar Yehuda S, Axlerod R, Toker O et al. The Association of Inflammatory Bowel Diseases with Autoimmune Disorders: A Report from the epi-IIRN. Journal of Crohn’s & colitis. 2019;13:324–329. https://doi.org/10.1093/ecco-jcc/jjy166.
Yates VM, Watkinson G, Kelman A. Further evidence for an association between psoriasis, Crohn’s disease and ulcerative colitis. Br J Dermatol. 1982;106:323–330. https://doi.org/10.1111/j.1365-2133.1982.tb01731.x.
Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. https://doi.org/10.1053/j.gastro.2005.06.021.
Weng X, Liu L, Barcellos LF, Allison JE, Herrinton LJ. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organization. The American journal of gastroenterology. 2007;102:1429–1435. https://doi.org/10.1111/j.1572-0241.2007.01215.x.
Moon JM, Lee JY, Koh SJ et al. Incidence of Psoriasis in Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Matched Cohort Study. Dermatology. 2021;237:330–337. https://doi.org/10.1159/000514030.
Ellinghaus D, Ellinghaus E, Nair RP et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet. 2012;90:636–647. https://doi.org/10.1016/j.ajhg.2012.02.020.
Liu JZ, van Sommeren S, Huang H et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nature genetics. 2015;47:979–986. https://doi.org/10.1038/ng.3359.
Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nature genetics. 2011/03/01 2011;43:246–252. doi:https://doi.org/10.1038/ng.764
Piruzian ES, Ishkin AA, Nikol'skaia TA, Abdeev RM, Bruskin SA. [The comparative analysis of psoriasis and Crohn disease molecular-genetical processes under pathological conditions]. Mol Biol (Mosk). Jan-Feb 2009;43:175–9.
Xi L, Garcet S, Ye Z, et al. A shared tissue transcriptome signature and pathways in psoriasis and ulcerative colitis. Sci Rep. Nov 17 2022;12:19740. doi:https://doi.org/10.1038/s41598-022-22465-w
Devaprasad A, Radstake T, Pandit A. Integration of Immunome With Disease-Gene Network Reveals Common Cellular Mechanisms Between IMIDs and Drug Repurposing Strategies. Front Immunol. 2021;12:669400. doi:https://doi.org/10.3389/fimmu.2021.669400
Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. https://doi.org/10.1136/gut.2009.199679.
RStudio Team (2016). RStudio: Integrated Development for R. RStudio I, Boston, MA URL http://www.rstudio.com/.
Wickham H, Averick M, Bryan J, al e. Welcome to the Tidyverse. Journal of Open Source Software. 2019;4:1686.
Sjoberg DWK, Curry M et al. Reproducible Summary Tables with the gtsummary Package. The R Journal. 2021;13:570–580.
Kassambara A. ggpubr: 'ggplot2' Based Publication Ready Plots. 2020;R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr
Terui H, Moroi R, Masamune A, Aiba S, Yamasaki K. Possible Efficacy of Vedolizumab, an Anti-α4β7 Integrin Antibody Palmoplantar Pustulosis. Case Rep Dermatol. 2023;15:22–25. https://doi.org/10.1159/000529080.
Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580–581. https://doi.org/10.17235/reed.2020.6817/2019.
Dai C, Jiang M, Sun MJ. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. The New England journal of medicine. Aug 3 2017;377:496. doi:https://doi.org/10.1056/NEJMc1707500
D’Haens G, Panés J, Louis E et al. Upadacitinib Was Efficacious and Well-tolerated Over 30 Months in Patients With Crohn’s Disease in the CELEST Extension Study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2022;20:2337-2346.e3. https://doi.org/10.1016/j.cgh.2021.12.030.
Valenzuela F, Korman NJ, Bissonnette R et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol. 2018;179:853–862. https://doi.org/10.1111/bjd.16798.
Zhang J, Tsai TF, Lee MG et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: A Phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88:36–45. https://doi.org/10.1016/j.jdermsci.2017.05.004.
Papp KA, Menter MA, Abe M et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–961. https://doi.org/10.1111/bjd.14018.
Bachelez H, van de Kerkhof PC, Strohal R et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet (London, England) 2015;386:552–561. https://doi.org/10.1016/s0140-6736(14)62113-9.
Acknowledgments
The authors acknowledge the Michigan Genomics Initiative participants, Precision Health at the University of Michigan, and the University of Michigan Medical School Data Office for Clinical and Translational Research for providing data storage, management, processing, and distribution services.
Funding
KCC reports departmental funds. JEG is supported by Taubman Medical Research Institute and P30-AR075043. ES is supported in part by R01 DK106621, R01 DK107904, R01DK128871, R01DK131787 and The University of Michigan Department of Internal Medicine. PDRH is supported by R01 DK125687, R01 DK118154, T32 DK062708.
Author information
Authors and Affiliations
Contributions
KC: study design, data analysis, results interpretation, drafting of the manuscript, critical review of the manuscript. JEG: results interpretation, critical review of the manuscript. ES: results interpretation, critical review of the manuscript. PDRH: study design, results interpretation, critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KC: No conflicts of interest. JEG: Reports advisory board roles for Eli Lilly, Almirall, Novartis, BMS, Astra-Zeneca, Boehringer Ingelheim, Sanofi, Galderma, AnaptysBio, and research grants from Almirall, Eli Lilly, Kyowa Kirin, and BMS/Celgene. ES: No conflicts of interest. PDRH: Reports personal fees from Abbvie, personal fees from Eli Lilly, and personal fees from Pfizer, outside of the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cushing, K., Gudjonsson, J.E., Speliotes, E. et al. An Assessment of Comparative Medication Durability in Inflammatory Bowel Disease Patients With and Without Co-morbid Psoriasis, Rheumatoid Arthritis, and/or Enteropathic Arthritis. Dig Dis Sci 68, 4001–4008 (2023). https://doi.org/10.1007/s10620-023-08062-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08062-5