Abstract
Background
Gastrointestinal (GI) symptoms are recognized sequelae of acute respiratory illness (ARI), but their prevalence is not well documented. Our study aim was to assess the incidence of GI symptoms in community ARI cases for persons of all ages and their association with clinical outcomes.
Methods
We collected mid-nasal swabs, clinical, and symptom data from Seattle-area individuals during the 2018–2019 winter season as part of a large-scale prospective community surveillance study. Swabs were tested by polymerase chain reaction (PCR) for 26 respiratory pathogens. Likelihood of GI symptoms given demographic, clinical, and microbiological covariates were analyzed with Fisher’s exact, Wilcoxon-rank-sum, and t-tests and multivariable logistic regression.
Results
In 3183 ARI episodes, 29.4% had GI symptoms (n = 937). GI symptoms were significantly associated with pathogen detection, illness interfering with daily life, seeking care for the illness, and greater symptom burden (all p < 0.05). Controlling for age, > 3 symptoms, and month, influenza (p < 0.001), human metapneumovirus (p = 0.004), and enterovirus D68 (p = 0.05) were significantly more likely to be associated with GI symptoms than episodes with no pathogen detected. Seasonal coronaviruses (p = 0.005) and rhinovirus (p = 0.04) were significantly less likely to be associated with GI symptoms.
Conclusion
In this community-surveillance study of ARI, GI symptoms were common and associated with illness severity and respiratory pathogen detection. GI symptoms did not track with known GI tropism, suggesting GI symptoms may be nonspecific rather than pathogen-mediated. Patients presenting with GI and respiratory symptoms should have respiratory virus testing, even if the respiratory symptom is not the primary concern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many common respiratory pathogens, including seasonal coronaviruses and influenza, can present with gastrointestinal (GI) symptoms like diarrhea, nausea, and vomiting [1]. However, for most respiratory pathogens, the incidence and severity of these symptoms are not well characterized [2, 3]. Given the significant burden of disease caused by primarily respiratory infections [4, 5], it is important to better characterize their diverse presentations to help guide rapid identification and appropriate clinical management.
Few prospective community-based surveillance studies of multiple respiratory pathogens and their symptomatology have been reported in the era of sensitive molecular diagnostics. However, previous studies suggest high rates of GI symptoms may be associated with coronavirus, influenza, and respiratory syncytial virus [1]. Most studies have been limited to hospitalized patients, children, or a single pathogen. Case–control studies of adults and children with diarrhea have identified pathogens with known respiratory manifestations such as adenovirus [6] and bocavirus [7] as causes of GI symptoms. There is a need for community-based sampling across age groups with broad pathogen testing and detailed symptomatic data collection to better understand the burden of GI symptoms in patients with respiratory infections.
The objective of this study was to assess the prevalence of GI symptoms in acute respiratory infections among cases detected through prospective community-based surveillance and the association between GI symptoms and clinical outcomes.
Methods
Population and Setting
Mid-nasal swabs and self-reported questionnaire data were collected from individuals with acute respiratory illness (ARI) between November 2018 and April 2019 as part of the Seattle Flu Study, a large-scale community surveillance study in Seattle, WA [8]. The study protocol and detailed methods have been previously published [8]. In brief, individuals were enrolled at community sites, which included clinics, workplaces, homeless shelters, daycares, and schools. Community members were also able to participate through an online self-initiated screening process.
Eligibility Criteria
Individuals of any age were eligible for participation if they had two or more of the following symptoms: fever, headache, sore throat, nausea or vomiting, rhinorrhea, fatigue, myalgia, dyspnea, diarrhea, ear pain or discharge, or rash. Subjects were also eligible to participate if they had new or worsening acute cough without additional symptoms. Individuals could not enroll more than once every 14 days or if symptoms were chronic.
Survey Methods
Participants aged 13 years and older then completed a symptom survey online accessed on their own device or on a tablet. Participants aged 7–12 years could have the survey completed by or in collaboration with their LAR. The LAR completed the symptom survey for individuals under age 7 years. Self-reported demographic, medical, and behavior data pertinent to the ARI episode were also collected as part of the survey. After survey completion, a mid-nasal swab was collected.
Sample Collection and Processing
Mid-nasal swabs from adults were self-collected and those from children were collected by a trained staff member at the time of enrollment. All used a nylon flocked swab that was transported in universal viral transport medium (Becton Dickinson, Franklin, NJ). Samples were tested by Taq-Man RT-PCR on the Open Array platform (ThermoFisher) for 26 respiratory pathogen targets (Supplemental Table 1). Because of high rates of asymptomatic carriage of S. pneumoniae, and low rates of other bacterial target detection, non-viral pathogens were excluded from the analyses.
Statistical Methods
GI symptoms were defined as self-reported diarrhea or a combined symptom of nausea or vomiting. Participants without respiratory tract symptoms (i.e., breathing trouble, cough, runny nose, sore throat, ear pain, n = 327) were excluded from our analysis, as they were not be considered to have ARI symptoms. Individuals with multiple pathogens detected were analyzed for each pathogen. Likelihood of GI symptoms given demographic, clinical, and microbiological covariates were analyzed with Fisher exact tests and t-tests or Wilcoxon-rank-sum test, as appropriate, for comparisons between groups. Two-tailed tests were used and p < 0.05 was considered statistically significant. Multivariable logistic regression was used to estimate the adjusted odds of GI symptoms by pathogen, controlling for reporting greater than three symptoms (a marker of disease severity), age, and month of sample collection. All analyses were conducted in R (version 3.5.0).
Results
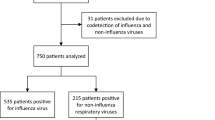
Of 3,183 ARI encounters with symptom data reported, 29.4% reported GI symptoms (n = 937, Table 1). Encounters for which the individual reported GI symptoms were more likely in individuals who were older (mean age 34.4 years vs. 29.0 years), those who had not received an influenza vaccination in the past year, and those who had public insurance (Table 1, all p < 0.001). Adults were more likely to report GI symptoms than children (30.8% in people ≥ 18 years vs. 26.2% in people < 18 years), though children were more likely to report nausea or vomiting (Supplemental Table 2) Episodes where the individual reported GI symptoms were more likely to have a respiratory pathogen detected (34.2% of episodes with GI symptoms had ≥ 1 pathogen detected vs. 30.3% of episodes without GI symptoms, p = 0.03, Table 2). Of encounters with a pathogen detected, 208 (20.3%) had detection of two or more respiratory pathogens (Supplemental Table 3). Children (age < 18 years) were more likely to have a pathogen detected than adults (p < 0.001). There was no significant difference in the likelihood of GI symptoms in encounters with one pathogen detected versus multiple pathogens detected (p = 0.79, Fig. 1).
Nausea or vomiting was the most common GI symptom reported (Table 2), and this was true in both children and adults (Supplemental Table 2). On average, individuals reporting GI symptoms were significantly more likely to report more symptoms overall (mean 6.9 symptoms reported for encounters with GI symptom vs. 4.1 no GI symptom, p < 0.001, Table 2). Episodes with GI symptoms were also significantly more likely to be associated with interference with daily life, visiting a doctor, and having received antibiotics for the illness episode (all p < 0.001).
Overall, 1001 of ARI encounters (31.4% of all encounters) had pathogen testing that was positive for at least one agent. Of these encounters with positive pathogen testing, individuals reported ≥ 1 GI symptom in 32.1% of them (n = 321). In ARI encounters without pathogens detected, individuals reported ≥ 1 GI symptom in 28.2% (n = 616), which was significantly low than in encounters with positive pathogen testing (p = 0.03). However, after controlling for age, and reporting ≥ 3 symptoms, there was no significant association between pathogen detection overall and GI symptoms (p = 0.21). In unadjusted analyses, enterovirus-D68, influenza, human metapneumovirus, and human parainfluenza were the pathogens most commonly associated with GI symptoms (Fig. 2).
Among episodes where a pathogen was identified, after controlling for potential confounders of age, reporting > 3 symptoms, and month of testing, episodes with seasonal coronavirus or rhinovirus were significantly less likely to be associated with GI symptoms than episodes with no pathogens detected, and influenza, human metapneumovirus, and enterovirus-D68 were significantly more likely to be associated with GI symptoms, relative to individuals with no pathogens detected (all p < 0.05, Fig. 3).
Adjusted odds ratios and 95% confidence intervals for presence of GI symptoms among individuals with acute respiratory illness, adjusted for presence of greater than three symptoms, number of pathogens detected, age, and month of illness. Odds ratios reflect odds of GI symptoms for individual infected with pathogen relative to individuals with acute respiratory illness but no pathogen detected. AdV adenovirus, CoV coronavirus, EV enterovirus, GI gastrointestinal, HBoV human bocavirus, hMPV human metapneumovirus, hPIV human parainfluenza virus, RSV respiratory syncytial virus, RV rhinovirus
Based on our finding that influenza was significantly associated with GI symptoms, we conducted an additional analysis to see whether influenza vaccination modified this relationship. Among episode where people tested positive for influenza, influenza vaccination was not associated with an increased risk of GI symptoms, controlling for number of symptoms reported, age, and month. In all episode (with and without pathogens detected), influenza vaccination was also not associated with a reduction in the risk of GI symptoms, controlling for number of symptoms reported, age, and month.
Discussion
In this community-based surveillance study of individuals of all ages, GI symptoms were a common feature of ARI. Episodes with GI symptoms were more severe, though they were not more likely to have a pathogen detected than in episodes without GI symptoms. The prevalence of GI symptoms varied significantly by pathogens. Infections with seasonal coronavirus and rhinovirus were slightly less likely to be associated with GI symptoms, and influenza, human metapneumovirus infection, and enterovirus-D68 were more likely to be associated with GI symptoms. There was no significant association between influenza vaccination and GI symptoms.
GI symptoms were reported in 32% of all episodes with an identified pathogen or pathogens on nasal swab. These episodes were also those where individuals were more likely to have sought medical care and to have received a course of antibiotics for their illness. This is congruent with that noted in the literature, with hospitalized individuals with ARI often reporting higher prevalence of GI symptoms than community-dwelling individuals with ARI [9, 10]. It is possible that for individuals who received antibiotics, this may have modified whether they had GI symptoms. However, we did not collect longitudinal data to determine whether GI symptoms pre-dated antibiotics. Overall, identifying community-based studies to compare our results to is difficult because there is heterogeneity among studies in both designs and results [3] and not all clinical presentations of pathogens included in our study have been rigorously studied [2].
We found that the prevalence of GI symptoms varied by pathogen, with seasonal coronaviruses and rhinovirus being less likely to have GI symptoms reported compared to no pathogen detection in adjusted analysis. Influenza, human metapneumovirus, and enterovirus-D68 were significantly more likely to have GI symptoms reported. In our study, GI symptoms were reported in 19% of encounters where coronaviruses were detected. This is significantly lower than the prevalence of GI symptoms reported in a prior clinic-based study, where they found 78% of individuals with ARI and coronaviruses detected reported GI symptoms [1]. Of note, 22% of encounters with rhinovirus detected had GI symptoms reported. This is less than the 33% reported by prior clinic-based work and higher than the 10% found in a prospective infant cohort [1, 10]. A meta-analysis of GI symptoms in influenza reported 3–30% of individuals had GI symptoms with differences between viral strains [3]. Enterovirus D68 has been reported to cause GI symptoms [11], but no community-level estimates of the incidence of these symptoms are available.
Interestingly, there was not a clear correlation between pathogens with known enteric tropism and GI symptoms. For example, some strains of coronaviruses have documented enteric tropism, but in our study the prevalence of GI symptoms in episodes with coronaviruses was lower than for respiratory syncytial virus, which does not replicate in the GI tract [12, 13]. One possible reason is under-ascertainment of seasonal coronavirus infections, as they are diverse and only two targets were included on our panel. However, another possibility is that the mechanisms of GI symptoms in ARI may be diverse, including direct infection of the GI tract and secondary damage from immune activation. This may vary not only by virus but by strain. For example, some influenza strains, like H5N1, can directly infect human tissue [14] while others cause GI damage by Th17-reated immune activation [15]. The latter mechanism may help explain why more severe cases of ARI were more likely to have GI symptoms reported in our study.
We found no association between influenza vaccination and GI symptoms overall or in individuals with PCR-confirmed influenza. Possible explanations for this include limited effectiveness of the 2018–2019 vaccine, inadequate study power to detect an effect, or a mechanism for GI symptoms that is independent of vaccine-related protection. The 2018–2019 influenza vaccine had only 29% efficacy across all age groups [16]. Assuming a 29% reduction in the incidence of GI symptoms, a baseline incidence of GI symptoms in in influenza infections of 42%, and an alpha of 0.05, we would have needed roughly 242 vaccinated individuals and 242 unvaccinated individuals with influenza to have 80% power—nearly double the number of influenza cases we identified in our study.
Strengths of our study include the description of clinical presentations of a wide range of viral respiratory pathogens in a community-based sample of both adults and children. Prior studies have mostly been conducted in healthcare settings, among children, or both. Because our sampling was not limited to a healthcare setting, we may have been able to capture more individuals with mild ARI and otherwise healthy individuals who may not typically seek medical care. As this study was conducted prior to the SARS-CoV-2 pandemic, the results are not affected by changes in infection prevention behaviors. Lastly, our study included a detailed symptom questionnaire that included non-respiratory symptoms, which allowed us to describe the diverse clinical manifestations of viral respiratory infections.
One major limitation of our study is that we did not collect stool specimens. Therefore, we may have missed cases of enteric pathogens presenting with respiratory symptoms. Enteric pathogens can be identified in patients with ARI symptoms, with 13% of episodes of ARI having enteric pathogens detected in one study [1]. An additional limitation is that data on antibiotic administration was collected at the same time as symptom data and did not specify whether there was a temporal relationship between antibiotics and subsequent GI symptoms that may not have been present at the start of the ARI episode. A further limitation is GI symptoms were included in the list of symptoms that identified eligible participants. It is possible that this may have inflated the proportion with GI symptoms, though we excluded individuals without respiratory symptoms from our analysis. Other limitations include convenience sampling in our population, which yielded a cohort that is not representative of the overall US population and under-sampled elderly individual; reliance on self-report for symptoms and care-seeking data; lack of follow up; lack of a comprehensive set of GI symptom questions; and possible missed infections that were not included on our panel.
This research raises several important questions for future research. These include the role of GI symptoms in spreading canonically respiratory pathogens, the predictive value of GI symptoms in determining who will go on to have severe illness from influenza, and the causal pathway by which respiratory pathogens lead to GI symptoms and whether this has other immunologic implications.
Conclusions
In this community-surveillance study for respiratory disease, GI symptoms were common. GI symptoms were associated with higher illness severity and more interference with daily activities. There was variation between the viruses as to their association with GI symptoms, even after adjusting for severity and other potential confounders. However, this did not track directly with known enteric viral tropism. This suggests that GI symptoms may be nonspecific responses to infection in many infections rather than pathogen-mediated. Patients presenting with GI and respiratory symptoms should have respiratory virus testing, even if the respiratory symptom is not the primary concern because presence of respiratory viruses may affect recommended isolation and treatment recommendations.
References
Minodier L, Masse S, Capai L et al. Clinical and virological factors associated with gastrointestinal symptoms in patients with acute respiratory infection: a two-year prospective study in general practice medicine. BMC Infect Dis. 2017;17:729.
Russell E, Ison MG. Parainfluenza virus in the hospitalized adult. Clin Infect Dis. 2017;65:1570–1576.
Minodier L, Charrel RN, Ceccaldi PE et al. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J. 2015;12:215.
Shi T, Denouel A, Tietjen AK et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2019;222:577–583.
Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210.
Qiu FZ, Shen XX, Li GX et al. Adenovirus associated with acute diarrhea: a case-control study. BMC Infect Dis. 2018;18:450.
Cheng WX, Jin Y, Duan ZJ et al. Human bocavirus in children hospitalized for acute gastroenteritis: a case-control study. Clin Infect Dis. 2008;47:161–167.
Chu HY, Boeckh M, Englund JA, et al. The Seattle Flu Study: a multi-arm community-based prospective study protocol for assessing influenza prevalence, transmission, and genomic epidemiology. medRxiv. 2020:2020.03.02.20029595.
Wang CYT, Ware RS, Lambert SB et al. Parechovirus A infections in healthy Australian children during the first 2-years of life: a community-based longitudinal birth cohort study. Clin Infect Dis. 2019;71:116–127.
Toivonen L, Schuez-Havupalo L, Karppinen S et al. Rhinovirus infections in the first 2 years of life. Pediatrics. 2016. https://doi.org/10.1542/peds.2016-1309.
Pham NTK, Thongprachum A, Baba T et al. A 3-month-old child with acute gastroenteritis with enterovirus D68 detected from stool specimen. Clin Lab. 2017;63:1269–1272.
Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055.
Su S, Wong G, Shi W et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502.
Shu Y, Li CK, Li Z et al. Avian influenza A(H5N1) viruses can directly infect and replicate in human gut tissues. J Infect Dis. 2010;201:1173–1177.
Wang J, Li F, Wei H et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397–2410.
Prevention CfDCa. US Flu VE Data for 2018–2019. Centers for Disease Control and Prevention, 2019.
Acknowledgments
We gratefully acknowledge the participating sites, individuals, and staff. We also thank the Seattle Flu Study investigators. The Seattle Flu Study was funded by Gates Ventures. The funder was not involved in the design of the study, does not have any ownership over the management and conduct of the study, the data, or the rights to publish. Award/Grant Number is not applicable. KLN was funded by NIH 5T32DK094775-10 and 1F32DK134043-01.
Funding
The Seattle Flu Study was funded by Gates Ventures (MB, JAE, HYC). KLN was funded by NIH 5T32DK094775-10 and 1F32DK134043-01. These funding sources were not involved in the design of the study and do not have any ownership over the management and conduct of the study, the data, the analysis, the rights to publish, or the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors have read and approved this manuscript and meet authorship criteria. Conceptualization: KLN, CRW, JKL, JAE, MB, HYC; data curation: KLN, CRW, JKL; funding acquisition: JAE, MB, HYC; formal analysis: KLN; investigation: KLN, CRW, JKL, JAE, MB, HYC; methodology: KLN, JAE, MB, HYC; project administration: CRW, JKL, JAE, MB, HYC; supervision: JAE, MB, HYC; visualization: KLN; writing (original draft): KLN; writing (review and editing): KLN, CRW, JKL, JAE, MB, HYC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose relevant to this publication.
Ethical approval
The Seattle Flu Study was approved by the University of Washington Human Subjects Institutional Review Board. All subjects or their LAR signed informed consent.
Informed consent
After screening all individuals for eligibility, informed consent was obtained directly from the individual or from a legally authorized representative (LAR) if they were less than 18 years old.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Newman, K.L., Wolf, C.R., Logue, J.K. et al. Nausea, Vomiting, and Diarrhea Are Common in Community-Acquired Acute Viral Respiratory Illness. Dig Dis Sci 68, 3383–3389 (2023). https://doi.org/10.1007/s10620-023-07976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07976-4