Abstract
Background
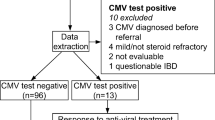
Diagnosis of cytomegalovirus (CMV) colitis in the setting of severe ulcerative colitis (UC) remains a clinical challenge. This study aimed to determine the utility of serum CMV polymerase chain reaction (PCR) as a non-invasive test for the diagnosis of CMV superinfection in patients hospitalized with UC.
Methods
This retrospective study included consecutive admitted patients with UC who had serum testing for CMV completed as part of standard hospital procedure and CMV colitis diagnosed by expert pathologists.
Results
Two hundred and six patients with UC were included; 13 patients (6%) had histologically confirmed CMV colitis. Eleven of 13 patients with CMV colitis (84%) and 3 of 193 (1.5%) patients without CMV colitis had a positive serum PCR test (p < 0.0001). ROC analysis showed that a CMV PCR level of 259 IU/mL had a sensitivity and specificity of 77% and 99%, respectively, for diagnosis of CMV colitis with an AUC of 0.9 (p < 0.0001). Serum CMV PCR level significantly correlated to the number of inclusion bodies on biopsy specimens with data available (n = 8) (r = 0.8, p = 0.02). CMV positivity did not predict the need for salvage therapy, admission or 1-year colectomy rates.
Conclusion
Serum CMV PCR has an excellent negative predictive value and demonstrates a strong correlation with CMV positivity on histology. This work supports a rationale for serum CMV PCR testing on admission to assess the risk of CMV colitis in patients with severe UC.

Similar content being viewed by others
Data availability
All original data are available from the authors upon request.
References:
Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12:S701–S710.
Siegmund B. Cytomegalovirus infection associated with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2017;2:369–376.
Kopylov U et al. Antiviral therapy in cytomegalovirus-positive ulcerative colitis: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:2695.
Shukla T, Singh S, Loftus EV, Bruining DH, McCurdy JD. Antiviral therapy in steroid-refractory ulcerative colitis with cytomegalovirus: systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2718–2725.
Jones A et al. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy forcytomegalovirus. Clin Gastroenterol Hepatol. 2015;13:949–955.
Clos-Parals A et al. Prognostic value of the burden of cytomegalovirus colonic reactivation evaluated by immunohistochemical staining in patients with active ulcerative colitis. J. Crohn’s Colitis 2019;13:385–388.
Zagórowicz E et al. Cytomegalovirus infection in ulcerative colitis is related to severe inflammation and a high count of cytomegalovirus-positive cells in biopsy is a risk factor for colectomy. J. Crohn’s Colitis 2016;10:1205–1211.
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413.
Pillet S et al. Infliximab does not worsen outcomes during flare-ups associated with cytomegalovirus infection in patients with ulcerative colitis. Inflamm Bowel Dis. 2015;21:1580–1586.
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
NAC, DMM: Study concept and design; NAC, MZ, MJA: Acquisition of data; NAC, NS, DMM: Analysis and interpretation of data; NAC: Drafting of manuscript; NS, DTR, DMM: Critical revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NAC, NS, MZ, MJA and DM: no conflicts of interest. DTR: Received grant support from Takeda; and has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences S.A., InDex Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Narrow River Mgmt, Pfizer, Prometheus Laboratories,Reistone, Takeda, and Techlab Inc. He is also co-founder of Cornerstones Health, Inc. and GoDuRn, LLC; on the Board of Trustees of the American College of Gastroenterology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, N.A., Zafer, M., Setia, N. et al. Serum Cytomegalovirus Polymerase Chain Reaction Test Is a Valuable Negative Predictor of Infection in Acute Severe Ulcerative Colitis. Dig Dis Sci 68, 897–901 (2023). https://doi.org/10.1007/s10620-022-07607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07607-4