Abstract
Background
Active Crohn’s disease increases the risk of strictures, fistulas, and abscesses. Less than 30% of patients with Crohn’s disease achieve endoscopic remission on any therapy. Tofacitinib may be a therapeutic option for patients with refractory Crohn’s disease.
Aims
We aimed to evaluate the safety and effectiveness of off-label tofacitinib for refractory Crohn’s disease.
Methods
We retrospectively assessed adverse events and clinical/endoscopic response after therapy.
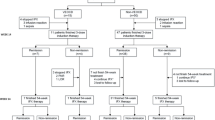
Results
Forty-four patients were included in the safety analysis and 35 were included in the clinical and/or endoscopic assessments. The mean age was 41.8 years and the mean disease duration was 17.4 years. All patients had prior biologic exposure. Adverse events were reported in 52.3% of patients; 13.6% had ≥ 1 serious adverse event after a median 54.6 weeks of treatment. Seventy percent achieved clinical response after a mean 29.4 (SD 15.1) weeks, and 33.3% achieved clinical remission after a mean 33.4 (SD 17.6) weeks of therapy. Endoscopic improvement occurred in 25.0%, endoscopic remission in 12.5%, and endoscopic healing in 4.2% of patients after a mean 52.0 (SD 15.0) weeks of therapy. The mean Simple Endoscopic Score in Crohn’s disease significantly improved from 23.1 ± 3.7 to 18.0 ± 13.7 after treatment (P = .02).
Conclusions
In the short term, tofacitinib appears well tolerated. The most common adverse event was minor infection. One serious infection and one colorectal cancer occurred. While half of patients reported adverse events, this likely reflects the severe refractory disease in this population and no new safety events were observed. Tofacitinib achieved clinical and endoscopic improvement in some patients with refractory Crohn’s disease. Further research is needed to understand the long-term safety and efficacy of tofacitinib in Crohn’s disease.


Similar content being viewed by others
References
Thia KT, Sandborn WJ, Harmsen WS et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–1155.
Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–721.
Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016;375:1946–1960.
Pérez-Jeldres T, Tyler CJ, Boyer JD et al. Targeting Cytokine Signaling and Lymphocyte Traffic via Small Molecules in Inflammatory Bowel Disease: JAK Inhibitors and S1PR Agonists. Front Pharmacol 2019;10:212.
Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–497.
Panes J, Sandborn WJ, Schreiber S et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–1059.
Rogler G. Efficacy of JAK inhibitors in Crohn’s disease. J Crohns Colitis 2020;14:S746-s754.
Bauer CM, Barnes EL, Herfarth HH. Tofacitinib in the treatment of Crohn’s-like disease of the pouch. Am J Gastroenterol 2020;115:2116–2117.
Fenster M, Alayo QA, Khatiwada A et al. Real-world effectiveness and safety of tofacitinib in Crohn's disease and IBD-U: a multicenter study from the TROPIC consortium. Clin Gastroenterol Hepatol 2020
Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz, Xeljanz XR (tofacitinib), in Drug Safety Communication. 2021. FDA: https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514.
Daperno M, D’Haens G, Van Assche G et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–512.
Sandborn WJ, Su C, Sands BE et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–1736.
Sandborn WJ, Panes J, D'Haens GR et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2018
Sandborn WJ, Panés J, Sands BE et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–1076.
Funding
This study did not receive specific funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SL, KC-S, AS, and KK. The first draft of the manuscript was written by SL and KC-S and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Scott Lee receives grant and research support from the following: AbbVie Pharmaceuticals, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., Bristol Myers Squibb Pharmaceuticals, Inc., Pfizer Pharmaceuticals, Inc., Atlantic Pharmaceuticals, Ltd., Gilead Sciences, Inc., Tetherex Pharmaceuticals, Arena Pharmaceuticals, Shield Therapeutics PLC and is a consultant for UCB Pharma, Cornerstones, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., and Eli Lilly Company. Kindra Clark-Snustad has been a consultant for Pfizer Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., AbbVie Pharmaceuticals, and Bristol Myers Squibb Pharmaceuticals, Inc. Kendra J. Kamp is supported, in part, by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Program, at the University of Washington (Grant Nr. T32DK007742). For the remaining authors no conflicts of interest were declared.
Ethical approval
Declarations: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the University of Washington Human Subjects Division.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-022-07448-1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S.D., Singla, A., Harper, J. et al. Tofacitinib Appears Well Tolerated and Effective for the Treatment of Patients with Refractory Crohn’s Disease. Dig Dis Sci 67, 4043–4048 (2022). https://doi.org/10.1007/s10620-022-07444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07444-5