Abstract
Background
Patients with SARS-CoV-2 who present with gastrointestinal symptoms have a milder clinical course than those who do not. Risk factors for severe COVID-19 disease include increased adiposity and sarcopenia.
Aims
To determine whether body composition risk factors are associated with worse outcomes among patients with gastrointestinal symptoms.
Methods
This was a retrospective study of hospitalized patients with COVID-19 who underwent abdominal CT scan for clinical indications. Abdominal body composition measures including skeletal muscle index (SMI), intramuscular adipose tissue index (IMATI), visceral adipose tissue index (VATI), subcutaneous adipose tissue index (SATI), visceral-to-subcutaneous adipose tissue ratio (VAT/SAT ratio), and liver and spleen attenuation were collected. The association between body composition measurements and 30-day mortality was evaluated in patients with and without gastrointestinal symptoms at the time of positive SARS-CoV-2 test.
Results
Abdominal CT scans of 190 patients with COVID-19 were evaluated. Gastrointestinal symptoms including nausea, vomiting, diarrhea, or abdominal pain were present in 117 (62%). Among patients without gastrointestinal symptoms, those who died had greater IMATI (p = 0.049), less SMI (p = 0.010), and a trend toward a greater VAT/SAT ratio. Among patients with gastrointestinal symptoms, those who died had significantly greater IMATI (p = 0.025) but no differences in other measures.
Conclusions
Among patients with COVID-19, those without gastrointestinal symptoms showed the expected associations between mortality and low SMI, high IMATI, and trend toward higher VAT/SAT ratio, but those with gastrointestinal symptoms did not. Future studies should explore the mechanisms for the altered disease course in patients with COVID-19 who present with gastrointestinal symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In coronavirus disease 2019 (COVID-19), obesity has emerged as a leading patient risk factor for severe clinical disease and mortality [1,2,3,4]. Among hospitalized patients with COVID-19, elevated body mass index (BMI) has been independently associated with increased risk for death or intubation [5].
BMI, which does not incorporate measures related to body fat and muscle stores, does not fully capture the increased health risks associated with adiposity. Adipose tissue distribution—specifically, increased visceral adiposity—is more closely associated with adverse clinical outcomes than BMI across multiple categories of disease, including stroke, heart disease, and cancer [6,7,8]. This relationship also appears to hold for visceral adiposity and outcomes related to sepsis, with a limited number of studies, suggesting that increased visceral adiposity is associated with a more severe COVID-19 clinical course [9, 10].
COVID-19 is primarily a respiratory illness, but gastrointestinal symptoms—such as nausea, vomiting, or change in bowel habits—appear in half of patients [11,12,13]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes COVID-19, enters host cells via the ACE-2 receptor, which is densely expressed on enteric epithelial cells and in the hepatobiliary system. The presence of gastrointestinal symptoms at disease onset has been associated with different disease course and disease severity than when such symptoms are absent [14,15,16,17]. Because patients with gastrointestinal symptoms seem to have a different phenotype of COVID-19, it is possible that risk factors for disease severity may vary between patients with or without gastrointestinal symptoms.
The primary aim of this study was to evaluate the relationship between body composition measures of obesity using abdominal computed tomography (CT) scans—including skeletal muscle, intramuscular adipose tissue, visceral adipose tissue, subcutaneous adipose tissue, and hepatic steatosis—and clinical outcomes related to COVID-19, and to determine whether this relationship differed based on the presence or absence of gastrointestinal symptoms.
Methods
Patient Population
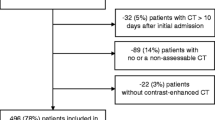
We performed a retrospective cohort study using data from the electronic medical record at New York Presbyterian-Columbia University Irving Medical Center. Adults > = 18 years of age were considered for the study if they were hospitalized for COVID-19 from March 6, 2020 (the start of the COVID-19 pandemic at our institution), to May 1, 2020. Patients were included if they had a positive SARS-CoV-2 test during or within 72 h before an inpatient admission and had an abdominal CT scan performed within 30 days before or after the SARS-CoV-2 test. If patients were tested more than once, the first positive SARS-CoV-2 test was used. This study was approved by the Columbia University Medical Center Institutional Review Board.
Gastrointestinal Symptoms
The presence or absence of gastrointestinal symptoms was evaluated at the time of positive SARS-CoV-2 test based on manual review of provider documentation at the time the respective test was performed. Gastrointestinal symptoms could include nausea or vomiting, abdominal pain, or new onset diarrhea or constipation. Respiratory signs/symptoms included cough, shortness of breath, or hypoxia.
Clinical Outcomes
Pertinent clinical outcomes, including admission to an intensive care unit, death, discharge to a hospice facility, and final hospital disposition, were ascertained from the electronic medical record, which interfaces with the national social security death index. The primary outcome of interest was death or discharge to hospice within 30 days after positive SARS-CoV-2 test. Secondary outcomes were admission to ICU within 30 days and a pooled outcome of admission to ICU or death/hospice within 30 days.
Abdominal CT Scans
Patients were included in the study only if an analyzable CT scan was available within 30 days of their first positive SARS-CoV-2 test. CT scans could be performed for any indication and could be performed before or after COVID-19 was diagnosed, provided they fell within the 30-day time frame. For example, a CT scan performed when a patient presented to the emergency department for diarrhea and was diagnosed with COVID-19 and an outpatient CT scan performed to stage malignancy weeks prior to presentation for COVID-19 would both meet criteria for the study, provided both scans were analyzable and fell with the 30-day window of COVID-19 diagnosis. To evaluate indications for CT scans, provider documentation immediately prior to the scan was reviewed. CT scan indications were classified as: suspected abdominal, intestinal, or hepatobiliary pathology; as part of multi-organ evaluation of fever or sepsis, often performed in combination with CT scan of the chest; suspected retroperitoneal hemorrhage; cancer diagnosis, staging, or surveillance; suspected genitourinary pathology such as for hematuria or suspected kidney stone; gastrointestinal bleeding; or other. If more than one abdominal CT scan was available, the scan closest to the date of SARS-CoV-2 test was used.
Body Composition Measurements
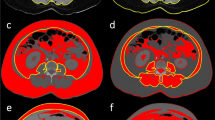
Body composition measurements for skeletal muscle (SM) tissue area, intramuscular adipose tissue (IMAT) area, visceral adipose tissue (VAT) area, and subcutaneous adipose tissue (SAT) area were taken on a single axial CT slice at the L3 vertebral level, using TomoVision sliceOmatic version 5.0 software (Quebec, Canada). All included CT scans were protocoled with standard slice thickness of 2.5–3.0 mm. The L3 vertebral level was selected due to prior evidence that this location provides the most accurate single-slice estimates of visceral adiposity [18,19,20,21]. Segmentation of muscle and adipose tissue compartments was performed according to the software specifications and as previously described, using tissue demarcation thresholds of 29 to 150 Hounsfield units (HU) for SM, − 190 to − 30 HU for IMAT, − 150 to − 50 HU for VAT, and − 190 to − 30 HU for SAT [22, 23]. All measures were normalized to height, to generate SM Index, IMAT Index, VAT Index, and SAT Index, respectively. VAT/SAT ratio was calculated by dividing non-normalized VAT area by SAT area.
The presence of hepatic steatosis was determined based on the measurement of liver and spleen attenuation from non-contrast CT scans as previously described [24, 25]. Liver and spleen attenuation in HU was calculated on a single axial CT slice using regions of interest (ROIs) between 80 and 100 mm2 in area, with two ROIs placed in the right lobe of the liver anteriorly and one ROI placed in the spleen. Absolute liver attenuation was defined as average attenuation of both right liver lobe ROIs; lower absolute liver attenuation is consistent with increased hepatic steatosis. Liver-to-spleen attenuation ratio was calculated by dividing the absolute liver attenuation by the spleen attenuation; hepatic steatosis was defined as liver/spleen attenuation ratio less than 1.
All scans were analyzed by a single reader (SS). A random selection of 19 scans (10% of the study population) were analyzed by a second reader (YRN) and demonstrated high inter-reader reliability, with intraclass correlations above 90% for all measures (Supplementary Table 1). SAT was excluded if any of the subcutaneous tissue was outside the field of the scan. Index measures were excluded if patient height was not available in the electronic medical record.
Co-variables
Patient clinical characteristics were extracted from the electronic medical record, including age, sex, race/ethnicity, body mass index (BMI), height, and the presence of specific medical comorbidities (hypertension, diabetes mellitus, or chronic liver disease) based on ICD codes. Laboratory values—including white blood cell (WBC) count, platelet count, aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, international normalized ratio (INR), D-dimer, lactate dehydrogenase (LDH), creatine phosphokinase (CPK), high-sensitivity troponin-T, lactate, and interleukin-6 (IL-6)—were extracted, and peak value for each laboratory measure in the time frame between 3 days prior to 14 days after SARS-CoV-2 test was included in the study.
Statistical Analyses
Continuous variables were summarized using medians, and categorical variables were summarized using proportions. Continuous variables were compared using rank-sum tests, and categorical variables were compared using Fisher’s exact tests. For validation of CT scan measurements, intraclass correlations were calculated using a two-way random effects model. Statistical calculations were performed using STATA version 16 (College Station, TX).
Results
Study Population
A total of 190 inpatients with COVID-19 were included in the study. Of these, 117 (62%) presented with gastrointestinal symptoms at the time of positive SARS-CoV-2 test (Supplementary Table 2). The median time between abdominal CT scan and SARS-CoV-2 test was zero days among patients with gastrointestinal symptoms (i.e., scan was performed on the same day as the test) and 7 days in patients without gastrointestinal symptoms. The most common indications for CT scan were suspicion for intestinal, hepatobiliary, or abdominal compartment pathology, as part of a multi-organ evaluation of fever/sepsis, or evaluation for retroperitoneal hemorrhage (Supplementary Table 3).
Gastrointestinal Symptoms
Clinical characteristics of study participants based on the presence or absence of gastrointestinal symptoms at presentation are shown in Table 1. There were no differences in age, sex, race/ethnicity, body mass index (BMI), or presence of respiratory symptoms between the two groups. While there were no statistically significant differences in the presence of major medical comorbidities in the two groups, there was a trend toward higher prevalence of diabetes mellitus among patients with gastrointestinal symptoms (47% versus 33% of patients without gastrointestinal symptoms; p = 0.06).
Clinical Outcomes
Of the 190 study patients, 46 (24%) were admitted to the ICU and 31 (16%) died or were discharged to hospice within 30 days after first positive SARS-CoV-2 test. Patients with gastrointestinal symptoms were significantly less likely to be admitted to the ICU, but rate of death or discharge to hospice within 30 days did not differ between those with versus without gastrointestinal symptoms (Fig. 1).
Body Composition Measures
Body composition parameters and metrics of hepatic steatosis were measured from abdominal CT scans (Fig. 2). SAT was excluded in 30 patients because the tissue area exceeded the boundaries of the scan, and SM and IMAT were excluded for one patient in whom segmentation of these compartments was not possible due to poor image quality (Supplementary Fig. 1). There were no significant differences in skeletal muscle index (SMI), intramuscular adipose tissue index (IMATI), visceral adipose tissue index (VATI), subcutaneous adipose tissue index (SATI), or VAT/SAT ratio between those with and without gastrointestinal symptoms. Although absolute liver attenuation did not differ significantly between those with and without gastrointestinal symptoms, hepatic steatosis based on liver-to-spleen attenuation ratio was present in significantly fewer patients with gastrointestinal symptoms compared to patients without (23% versus 38% of patients, respectively; p = 0.04).
Relationship Between Body Composition and Clinical Outcomes Based on the Presence of Gastrointestinal Symptoms
Body composition measures in patients who did or did not meet the primary clinical outcome of death or discharge to hospice within 30 days are shown in Table 2. Among all patients either with or without gastrointestinal symptoms, those who died had a significantly higher IMATI compared to those who did not die. Only among patients without gastrointestinal symptoms was SMI associated with mortality: SMI was 42.7 (IQR 35.0–50.4) among those who died compared to 32.3 (IQR 30.1–41.5) among those who did not die (Table 2). Among patients without gastrointestinal symptoms, there was a nonsignificant trend toward elevated VAT/SAT ratio in those who died, which was not seen in patients with gastrointestinal symptoms. No other measures differed with respect to clinical outcomes comparing those with versus those without gastrointestinal symptoms.
Inflammatory Markers
Associations between body composition and serologic measurements, which provide additional information about disease severity, were evaluated in patients with and without gastrointestinal symptoms. Patients with gastrointestinal symptoms had significantly lower peak levels of WBC, ESR, transaminases (AST and ALT), D-dimer, LDH, and high-sensitivity troponin-T compared to patients without gastrointestinal symptoms (Table 3).
Discussion
Obesity—typically measured by BMI—is a risk factor for severe disease and poor clinical outcomes in patients with COVID-19 [1, 2, 4, 26]. Prior studies suggest that patients with prominent gastrointestinal symptoms at the time of COVID-19 diagnosis have better outcomes compared to patients who present with isolated respiratory symptoms [14,15,16,17]. The intersection of obesity and gastrointestinal symptoms in COVID-19 was the focus of this retrospective cohort study of 190 patients with COVID-19 who underwent abdominal CT scan for clinical indications. The study findings support the conclusion that gastrointestinal symptoms at the time of COVID-19 diagnosis mark a fundamentally different and milder clinical course, with lower rates of ICU admission and less aberrant laboratory values seen in patients with gastrointestinal symptoms. Less clear is the relationship between standard measures of body composition and adverse outcomes in COVID-19. High intramuscular adipose tissue index (IMATI, a measure of the fat content within muscle) was associated with death among all patients, with and without gastrointestinal symptoms. On the other hand, low skeletal muscle index (SMI, a measure of skeletal muscle mass) was only associated with death among those without gastrointestinal symptoms. Surprisingly, other common measures of body composition—including the visceral adipose tissue index (VATI, representing the amount of visceral fat), which has been linked with systemic inflammation—were not associated with death. This remained true even when the VATI was corrected for subcutaneous adipose tissue (VAT/SAT ratio) and was true regardless of the presence or absence of gastrointestinal symptoms.
A limited number of prior studies have evaluated cross-sectional adiposity in patients with COVID-19, and several have identified an association between increased VAT or VAT/SAT ratio and ICU admission or need for mechanical ventilation [27,28,29,30,31,32,33]. Only one of these investigated IMATI and found a relationship between IMATI and COVID-19 outcomes. Low muscle mass and intramuscular adiposity are associated with poor clinical outcomes in other diseases. For example, high IMAT content, low SM mass, and high VAT/SAT ratio are associated with mortality following liver transplantation and with risk of death or delisting prior to lung transplantation [34, 35]. Among patients with liver disease, reduced SM mass is associated with increased severity of liver injury, increased rate of complications such as infections or encephalopathy, and increased mortality [36,37,38]. In the context of sepsis and critical illness, higher IMAT content and lower SM mass are associated with increased ICU mortality and overall 30-day mortality [39]. Our results are consistent with these findings related to muscle mass/adiposity, but differ with respect to visceral fat.
The finding that VATI, a more accurate measure of obesity than BMI, was not associated with death in patients with COVID-19 is surprising. First, it is possible that the association between BMI and poor clinical outcomes in COVID-19 is unrelated to adipose tissue distribution; while an association between VAT and disease severity has been identified in prior studies, these studies used small sample sizes and varying metrics of adiposity quantification. Second, the gut microbiome is altered among patients with COVID-19, and the presence of gastrointestinal symptoms may reflect intestinal differences in SARS-CoV-2 viral load within the bowel [40,41,42,43,44]. If this is the case, the relationship between sarcopenia (muscle mass and intramuscular adiposity) and clinical outcomes in COVID-19 may be related to a microbiome-mediated “gut-muscle axis” [45]. Skeletal muscle wasting is mediated in part by inflammatory cytokines, including tumor necrosis factor (TNF)-α and nuclear factor (NF)-κB [46]. In our study, subjects with gastrointestinal symptoms had lower serologic inflammatory markers and did not show the expected association between SM index and mortality. It is possible that the physiology represented by the presence of gastrointestinal symptoms involves a reduced inflammatory response to COVID-19. Finally, it must be recognized that patients who underwent abdominal CT scans during the height of the COVID-19 pandemic—when access to imaging, even in the hospital, was highly limited—reflect a unique patient population.
Our study has limitations. While the majority of abdominal CT scans in our study were performed at the time of initial COVID-19 diagnosis, some scans were performed earlier or later within the included timeframe, and it is possible that body composition measures differed based on timing of CT scan during the disease course. SAT measurements were excluded if the patient’s SAT extended past the scan window, so we were unable to evaluate SATI and VAT/SAT ratio in patients with the highest SAT. Finally, it is possible that patients who underwent abdominal CT scans were dissimilar from patients in the general population of hospitalized patients with COVID-19, limiting generalizability of our findings. There are also study strengths. To our knowledge, ours is the largest study to date of body composition measures in patients with COVID-19. Body composition measurements were taken according to established, validated methods, and inter-reader consistency was confirmed, demonstrating reliability. These measures provide more accurate, specific information about body composition than BMI and may be less variable over the course of illness than measured weights.
Our findings are consistent with prior reports that patients with COVID-19 and gastrointestinal symptoms have improved outcomes compared to patients without gastrointestinal symptoms; whether the effect of adiposity differs based on gastrointestinal symptoms, and how this may impact outcomes, was less clear. Specifically, patients without gastrointestinal symptoms showed the expected associations between mortality and low SMI, high IMATI, and trend toward high VAT/SAT ratio, but this pattern was not observed in patients with gastrointestinal symptoms. Future studies are needed to elucidate the mechanisms underlying the altered disease course in patients with COVID-19 who present with gastrointestinal symptoms.
References
Simonnet A, Chetboun M, Poissy J et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195–1199.
Lighter J, Phillips M, Hochman S et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897.
Goyal P, Choi JJ, Pinheiro LC et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374.
Cai Q, Chen F, Wang T et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398.
Anderson MR, Geleris J, Anderson DR et al. Body mass index and risk of intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790.
Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012;55:2622–2630.
Fox CS, Massaro JM, Hoffmann U et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48.
Kim JH, Choi KH, Kang KW et al. Impact of visceral adipose tissue on clinical outcomes after acute ischemic stroke. Stroke. 2019;50:448–454.
Lee JGH, Genga KR, Pisitsak C et al. Survival benefit of a low ratio of visceral to subcutaneous adipose tissue depends on LDL clearance versus production in sepsis. Crit Care. 2018;22:58.
Foldi M, Farkas N, Kiss S et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity (Silver Spring). 2021;29:521–528.
Nobel YR, Phipps M, Zucker J et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159:373–375.
Sultan S, Altayar O, Siddique SM et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334.
Cholankeril G, Podboy A, Aivaliotis VI et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: early experience from California. Gastroenterology. 2020;159:775–777.
Dong ZY, Xiang BJ, Jiang M, Sun MJ, Dai C. The prevalence of gastrointestinal symptoms, abnormal liver function, digestive system disease and liver disease in COVID-19 infection: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:67–76.
Redd WD, Zhou JC, Hathorn KE et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765–767.
Aziz M, Haghbin H, Lee-Smith W, Goyal H, Nawras A, Adler DG. Gastrointestinal predictors of severe COVID-19: systematic review and meta-analysis. Ann Gastroenterol. 2020;33:615–630.
Livanos AE, Jha D, Cossarini F et al. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450.
Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 2010;34:781–787.
Zopfs D, Theurich S, Grosse Hokamp N et al. Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol. 2020;30:1701–1708.
Demerath EW, Shen W, Lee M et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85:362–368.
Shen W, Punyanitya M, Chen J et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (Lond). 2007;31:763–769.
Aubrey J, Esfandiari N, Baracos VE et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–497.
Brown JC, Caan BJ, Prado CM et al. Body composition and cardiovascular events in patients with colorectal cancer: a population-based retrospective cohort study. JAMA Oncol. 2019;5:967–972.
Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19:811–818.
Torgersen J, So-Armah K, Freiberg MS et al. Comparison of the prevalence, severity, and risk factors for hepatic steatosis in HIV-infected and uninfected people. BMC Gastroenterol. 2019;19:52.
Anderson MR, Geleris J, Anderson DR et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790.
Battisti S, Pedone C, Napoli N et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43:e129–e130.
Deng M, Qi Y, Deng L et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring). 2020;28:1815–1825.
Petersen A, Bressem K, Albrecht J et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
Watanabe M, Caruso D, Tuccinardi D et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319.
Yang Y, Ding L, Zou X et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity (Silver Spring). 2020;28:2040–2048.
Pediconi F, Rizzo V, Schiaffino S et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes Res Clin Pract. 2021;15:89–92.
Favre G, Legueult K, Pradier C et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440.
Hamaguchi Y, Kaido T, Okumura S et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101:565–574.
Anderson MR, Easthausen I, Gallagher G et al. Skeletal muscle adiposity and outcomes in candidates for lung transplantation: a lung transplant body composition cohort study. Thorax. 2020;75:801–804.
Al-Azzawi Y, Albo B, Fasullo M et al. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2020;32:733–738.
Hara N, Iwasa M, Sugimoto R et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55:863–870.
Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1229–1244.
Loosen SH, Schulze-Hagen M, Pungel T et al. Skeletal muscle composition predicts outcome in critically Ill patients. Crit Care Explor. 2020;2:e0171.
Cheung KS, Hung IFN, Chan PPY et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95.
Chen Y, Chen L, Deng Q et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833–840.
Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18:269–283.
Zuo T, Zhang F, Lui GCY et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955.
Zuo T, Liu Q, Zhang F et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284.
Ticinesi A, Mancabelli L, Tagliaferri S et al. The gut-muscle axis in older subjects with low muscle mass and performance: a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing. Int J Mol Sci. 2020;21:8946.
Thoma A, Lightfoot AP. NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol. 2018;1088:267–279.
Funding
YRN: NIH T32 DK083256-12. MRA: NIH K23 HL150280. JLS: Doris Duke Physician Scientist Fellowship and Columbia Integrated Program in Infectious Diseases Research (NIH T32 AI100852).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nobel, Y.R., Su, S.H., Anderson, M.R. et al. Relationship Between Body Composition and Death in Patients with COVID-19 Differs Based on the Presence of Gastrointestinal Symptoms. Dig Dis Sci 67, 4484–4491 (2022). https://doi.org/10.1007/s10620-021-07324-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07324-4