Abstract
Background
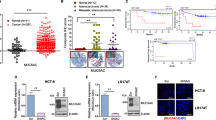
Mucin 16 (MUC16), a cell surface-associated mucin, has been implicated to be upregulated in a large repertoire of malignances. However, its function in the pathogenesis of colorectal cancer (CRC) is unknown.
Aims
Here, we explored the regulatory role of MUC16 in CRC.
Methods
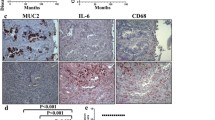
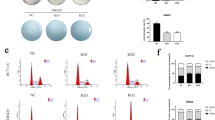
First, tumor and paracancerous tissues, and serum samples from 162 CRC patients, peripheral blood samples from 48 healthy volunteers and 72 benign colorectal patients were collected. The correlation between the MUC16 expression and the clinical phenotypes of the patients was analyzed. Subsequently, HCT116 and SW480 cells with deletion of MUC16 were established to detect changes in the growth and metastatic capacities of CRC cells. The genes with the highest correlation with MUC16 were predicted by bioinformatics, and their binding relationships were detected by Co-IP and double-labeled immunofluorescence, followed by functional rescue experiments.
Results
Overexpression of MUC16 in CRC patients was positively correlated with serum biomarkers and poor prognosis of patients. It was demonstrated by in vitro and in vivo experiments that knocking-down the expression of MUC16 could significantly inhibit the growth and metastasis of CRC cells. MUC16 activated janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) by interacting with JAK2. Further overexpression of JAK2 in cells with poor expression of MUC16 revealed a significant increase in the proliferative and metastatic capacities of CRC cells.
Conclusions
MUC16 contributes to the development and progression of CRC by binding to JAK2, thereby promoting phosphorylation of JAK2 and further activating STAT3 phosphorylation.






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Hull R, Francies FZ, Oyomno M, Dlamini Z. Colorectal cancer genetics, incidence and risk factors: in search for targeted therapies. Cancer Manag Res 2020;12:9869–9882.
Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786–6808.
Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885.
O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169.
Das S, Batra SK. Understanding the unique attributes of MUC16 (CA125): potential implications in targeted therapy. Cancer Res. 2015;75:4669–4674.
Theriault C, Pinard M, Comamala M et al. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol. 2011;121:434–443.
Rump A, Morikawa Y, Tanaka M et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198.
Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation. Cells. 2019;8.
He HL, Lee YE, Liang PI et al. Overexpression of JAK2: a predictor of unfavorable prognosis for nasopharyngeal carcinoma. Future Oncol. 2016;12:1887–1896.
Lakshmanan I, Ponnusamy MP, Das S et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012;31:805–817.
Jung G, Hernandez-Illan E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17:111–130.
Bruney L, Conley KC, Moss NM, Liu Y, Stack MS. Membrane-type I matrix metalloproteinase-dependent ectodomain shedding of mucin16/ CA-125 on ovarian cancer cells modulates adhesion and invasion of peritoneal mesothelium. Biol Chem. 2014;395:1221–1231.
Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99.
Chen Q, Li D, Ren J, Li C, Xiao ZX. MUC1 activates JNK1 and inhibits apoptosis under genotoxic stress. Biochem Biophys Res Commun. 2013;440:179–183.
Das S, Rachagani S, Sheinin Y et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene. 2016;35:2645–2654.
Reynolds IS, Fichtner M, McNamara DA, Kay EW, Prehn JHM, Burke JP. Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev. 2019;38:237–257.
Sheng YH, He Y, Hasnain SZ et al. MUC13 protects colorectal cancer cells from death by activating the NF-kappaB pathway and is a potential therapeutic target. Oncogene. 2017;36:700–713.
Zhu X, Long X, Luo X, Song Z, Li S, Wang H. Abrogation of MUC5AC expression contributes to the apoptosis and cell cycle arrest of colon cancer cells. Cancer Biother Radiopharm. 2016;31:261–267.
Streppel MM, Vincent A, Mukherjee R et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755–1763.
Liang C, Qin Y, Zhang B et al. Oncogenic KRAS targets MUC16/CA125 in pancreatic ductal adenocarcinoma. Mol Cancer Res. 2017;15:201–212.
Chen X, Li X, Wang X, Zhu Q, Wu X, Wang X. MUC16 impacts tumor proliferation and migration through cytoplasmic translocation of P120-catenin in epithelial ovarian cancer cells: an original research. BMC Cancer. 2019;19:171.
Akita K, Tanaka M, Tanida S, Mori Y, Toda M, Nakada H. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial cancer cells reduces cell-cell adhesion. Eur J Cell Biol. 2013;92:257–263.
Comamala M, Pinard M, Theriault C et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer. 2011;104:989–999.
Lai F, Deng W, Fu C, Wu P, Cao M, Tan S. Long non-coding RNA SNHG6 increases JAK2 expression by targeting the miR-181 family to promote colorectal cancer cell proliferation. J Gene Med. 2020;22:e3262.
Li S, Xu Z, Guo J, Zheng J, Sun X, Yu J. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J Cell Mol Med. 2020.
Zhang ZH, Li MY, Wang Z et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine. 2020;68:153172.
Zhao T, Li Y, Zhang J, Zhang B. PD-L1 expression increased by IFN-gamma via JAK2-STAT1 signaling and predicts a poor survival in colorectal cancer. Oncol Lett. 2020;20:1127–1134.
Gupta BK, Maher DM, Ebeling MC et al. Functions and regulation of MUC13 mucin in colon cancer cells. J Gastroenterol. 2014;49:1378–1391.
Shen H, Guo M, Wang L, Cui X. MUC16 facilitates cervical cancer progression via JAK2/STAT3 phosphorylation-mediated cyclooxygenase-2 expression. Genes Genomics. 2020;42:127–133.
Felder M, Kapur A, Gonzalez-Bosquet J et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129.
Lakshmanan I, Salfity S, Seshacharyulu P et al. MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by suppressing p53. Clin Cancer Res. 2017;23:3906–3917.
Xu Y, Lv SX. The effect of JAK2 knockout on inhibition of liver tumor growth by inducing apoptosis, autophagy and anti-proliferation via STATs and PI3K/AKT signaling pathways. Biomed Pharmacother. 2016;84:1202–1212.
Acknowledgments
The authors would like to thank the National Clinical Key Specialty Construction Project (2013-544), the Anhui Natural Science Foundation Project (1408085MKL70) and the grants of the Scientific Research of BSKY (XJ201935).
Funding
This work was supported by the National Clinical Key Specialty Construction Project (2013–544), the Anhui Natural Science Foundation Project (1408085MKL70) and the grants of the Scientific Research of BSKY (XJ201935).
Author information
Authors and Affiliations
Contributions
ZNL is the guarantor of integrity of the entire study and contributed to the concepts; YMG and XHL contributed to the design and definition of intellectual content of this study; LBZ, XHC, HJ, YH and YFZ contributed to the experimental studies, data acquisition and statistical analysis; TTX, WSY and QH contributed to the source, validation and methodology. All authors contributed to the manuscript preparation, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no potential conflicts of interest.
Ethics approval
The research was permitted by the Ethics Committee of the Second Hospital of Anhui Medical University, and was in accordance with the Declaration of Helsinki. All participants involved in this study signed an informed consent. All animal experiments were performed in accordance with the institutional guidelines and were approved by the Laboratory Animal Center of the Second Hospital of Anhui Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Gu, Y., Li, X. et al. Mucin 16 Promotes Colorectal Cancer Development and Progression Through Activation of Janus Kinase 2. Dig Dis Sci 67, 2195–2208 (2022). https://doi.org/10.1007/s10620-021-07004-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07004-3