Abstract
Background
Significant disparities in hepatitis C (HCV) treatment existed in the interferon treatment era, such that patients with mental health and substance use disorders were less likely to be treated. We aimed to evaluate whether these perceptions continue to influence HCV treatment decisions.
Methods
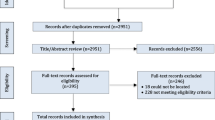
We e-mailed HCV providers a survey to assess their perceptions of barriers to HCV treatment adherence and initiation. We assessed the frequency of perceived barriers and willingness to initiate HCV treatment in patients with these barriers. We identified a group of providers more willing to treat patients with perceived barriers to adherence and determined the associated provider characteristics using Spearman’s rho and Wilcoxon rank-sum tests.
Results
A total of 103 providers (29%) responded to the survey. The most commonly endorsed perceived barriers to adherence were homelessness (65%), ongoing drug (58%), and ongoing alcohol use (33%). However, 90%, 68%, and 90% of providers were still willing to treat patients with these comorbidities, respectively. Ongoing drug use was the most common reason providers were never or rarely willing to initiate HCV treatment. Providers who were less willing to initiate treatment more frequently endorsed patient-related determinants of adherence, while providers who were more willing to initiate treatment more frequently endorsed provider-based barriers to adherence (e.g., communication).
Conclusions
Most responding providers were willing to initiate HCV treatment in all patients, despite the presence of perceived barriers to adherence or previous contraindications to interferon-based treatments. Ongoing substance use remains the most prominent influencer in the decision not to treat.





Similar content being viewed by others
References
Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US states and the district of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1:e186371.
Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88–96.
Lam BP, Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Ther Adv Gastroenterol. 2015;8:298–312.
Chainuvati S, Khalid SK, Kancir S, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat. 2006;13:235–241.
Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607.
Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221.
Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir–elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13.
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493.
Sustained virologic response in veterans in VHA care starting DAA therapy in 2014 or later for the Nation. Accessed January 6, 2019.
World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. 2016; http://www.who.int/iris/handle/10665/206453. Accessed March 14, 2019.
Rossi C, Young J, Martel-Laferriere V, et al. Direct-acting antiviral treatment failure among hepatitis C and HIV-coinfected patients in clinical care. Open Forum Infect Dis. 2019;6(3):ofz055.
Soriano V, Vispo E, Poveda E, Labarga P, Barreiro P. Treatment failure with new hepatitis C drugs. Expert Opin Pharmacother. 2012;13:313–323.
Benitez-Gutierrez L, Barreiro P, Labarga P, et al. Prevention and management of treatment failure to new oral hepatitis C drugs. Expert Opin Pharmacother. 2016;17:1215–1223.
Buti M, Esteban R. Management of direct antiviral agent failures. Clin Mol Hepatol. 2016;22:432–438.
Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933–1943. https://doi.org/10.1007/s10620-017-4608-9.
Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–266.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
Palmer RE, Carrell DS, Cronkite D, et al. The prevalence of problem opioid use in patients receiving chronic opioid therapy: computer-assisted review of electronic health record clinical notes. Pain. 2015;156:1208–1214.
Grebely J, Alavi M, Micallef M, et al. Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: the ETHOS Study. Addiction. 2016;111:311–319.
Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153–161.
Read P, Lothian R, Chronister K, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy. 2017;47:209–215.
Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108:175–181.
Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39:130–137.
Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101–109.
Rogal SS, Arnold RM, Chapko M, et al. The patient-provider relationship is associated with hepatitis C treatment eligibility: a prospective mixed-methods cohort study. PLoS ONE. 2016;11:e0148596.
Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus among homeless and nonhomeless United States veterans. Clin Infect Dis. 2017;65:252–258.
Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev.. 2015;37:131–143.
Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100(8):1772–1779.
Mishra G, Sninsky C, Roswell R, Fitzwilliam S, Hyams KC. Risk factors for hepatitis C virus infection among patients receiving health care in a Department of Veterans Affairs hospital. Dig Dis Sci. 2003;48:815–820. https://doi.org/10.1023/A:1022865515735.
Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Engagement in the hepatitis C care cascade among homeless veterans, 2015. Public Health Rep. 2017;132:136–139.
Rich ZC, Chu C, Mao J, et al. Facilitators of HCV treatment adherence among people who inject drugs: a systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health. 2016;16:994.
Chaiyachati KH, Hubbard RA, Yeager A, et al. Association of rideshare-based transportation services and missed primary care appointments: a clinical trial. JAMA Intern Med. 2018;178:383–389.
Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32.
Acknowledgments
The contents of this article are the views of the authors alone and do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding
This study was funded by an investigator-initiated research grant from Gilead Sciences to the institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, G., Patel, K., Moghe, A. et al. Provider Perceptions of Hepatitis C Treatment Adherence and Initiation. Dig Dis Sci 65, 1324–1333 (2020). https://doi.org/10.1007/s10620-019-05877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05877-z