Abstract
Background and Aim
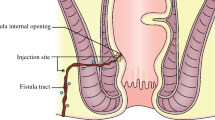
The introduction of mesenchymal stromal cells (MSCs) has changed the management of Crohn’s fistula, while it remains controversial. The aim of this study was to provide an overview of efficacy and optimum state of MSCs treatment on Crohn’s fistula.
Methods
Studies reporting MSCs treatment on Crohn’s fistula were searched and included. A fixed-effects model was used to assess the efficacy of MSCs, and outcomes of healing and recurrence were used to evaluate the best states of MSCs intervention.
Results
Fourteen articles were enrolled (n = 477). Pooled analysis showed MSCs had a significant efficacy compared to other treatments [risk difference: 0.21 (0.09, 0.32), P = 0.000]. Notably, after MSCs treatment, the group of Crohn’s disease activity index (CDAI) baseline >150 group had a higher healing rate (HR) and a clinical response (a change in CDAI of >50 points) (79.17 ± 8.78 vs. 47.54 ± 15.90, P = 0.011) compared to CDAI baseline of <150. The duration time of CD and fistulas had a negative correlation with HR accompanied by MSC therapy (r = −0.900, −0.925). Then, a moderate dose MSCs (2–4 × 107 cells/ml) had a higher HR (80.07%) and lower recurrence rate (RR 13.98%) compared to other dosages. Moreover, adipose-derived MSCs therapy had an advantage over bone marrow-derived MSCs in terms of low RR (7.4 ± 4.28 vs. 13.39 ± 0.89).
Conclusions
The evidence supported the effect of MSCs at a more appropriate time of Crohn’s fistula. And CDAI baseline (the points >150) has been a candidate for evaluating effectiveness of MSCs application on Crohn’s fistula.






Similar content being viewed by others
References
American Gastroenterological Association medical position statement. perianal Crohn’s disease. Gastroenterology. 2003;125:1503–1507.
Ardizzone S, Porro GB. Perianal Crohn’s disease: overview. Dig Liver Dis. 2007;39:957–958.
Peloquin JM, Goel G, Villablanca EJ, Xavier RJ. Mechanisms of pediatric inflammatory bowel disease. Annu Rev Immunol. 2016;34:31–64.
Bansal P, Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. Am J Gastroenterol. 1996;91:44–48.
Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729.
Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–1104.
von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839–855.
Algaba A, Guerra I, Castano A, et al. Risk of cancer, with special reference to extra-intestinal malignancies, in patients with inflammatory bowel disease. World J Gastroenterol. 2013;19:9359–9365.
Beaugerie L, Sokol H, Seksik P. Noncolorectal malignancies in inflammatory bowel disease: more than meets the eye. Dig Dis (Basel, Switzerland). 2009;27:375–381.
Katsanos KH, Tatsioni A, Pedersen N, et al. Cancer in inflammatory bowel disease 15 years after diagnosis in a population-based European collaborative follow-up study. J Crohn’s Colitis. 2011;5:430–442.
Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444.
Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (London, England). 2008;371:1579–1586.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822.
Alvaro-Gracia JM, Jover JA, Garcia-Vicuna R, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2016.
Jauregui-Amezaga A, Rovira M, Marin P, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65:1456–1462.
Lin R, Ding Z, Ma H, et al. In vitro conditioned bone marrow-derived mesenchymal stem cells promote de novo functional enteric nerve regeneration, but not through direct-transdifferentiation. Stem Cells (Dayton, Ohio). 2015;33:3545–3557.
Lin R, Ma H, Ding Z, et al. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22:2836–2848.
Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423.
Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149:918.e916–927.e916.
Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Exp Opin Biol Ther. 2008;8:1417–1423.
Sanz-Baro R, Garcia-Arranz M, Guadalajara H, de la Quintana P, Herreros MD, Garcia-Olmo D. First-in-human case study: pregnancy in women with Crohn’s perianal fistula treated with adipose-derived stem cells: a safety study. Stem Cells Transl Med. 2015;4:598–602.
Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long-term follow-up of CD fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin Proc. 2015;90:747–755.
de la Portilla F, Alba F, Garcia-Olmo D, Herrerias JM, Gonzalez FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323.
Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73:139–149.
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research ed). 2003;327:557–560.
Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71.
Knyazev OV, Parfenov AI, Shcherbakov PL, Ruchkina IN, Konoplyannikov AG. Cell therapy of refractory Crohn’s disease. Bull Exp Biol Med. 2013;156:139–145.
Garcia-Arranz M, Dolores Herreros M, Gonzalez-Gomez C, et al. Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I–IIa clinical trial. Stem Cells Trans Med. 2016.
Garcia-Olmo D, Guadalajara H, Rubio-Perez I, Herreros MD, de-la-Quintana P, Garcia-Arranz M. Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol. 2015;21:3330–3336.
Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595–600.
Mizushima T, Takahashi H, Takeyama H, et al. A clinical trial of autologous adipose-derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg Today. 2016;46:835–842.
Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798.
Cho YB, Park KJ, Yoon SN, Song KH, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4:532–537.
Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells (Dayton, Ohio). 2013;31:2575–2581.
Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279–285.
Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86.
Park KJ, Ryoo SB, Kim JS, et al. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis. 2016;18:468–476.
Panes J, Garcia-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet (London, England). 2016;388:1281–1290.
Garcia-Olmo D, Herreros D, Pascual M, et al. Treatment of enterocutaneous fistula in Crohn’s disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24:27–30.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Levy C, Tremaine WJ. Management of internal fistulas in Crohn’s disease. Inflamm Bowel Dis. 2002;8:106–111.
Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in CD. A meta-analysis. Ann Intern Med. 1995;123:132–142.
Hermann J, Eder P, Banasiewicz T, Matysiak K, Lykowska-Szuber L. Current management of anal fistulas in Crohn’s disease. Prz Gastroenterol. 2015;10:83–88.
Akiba RT, Rodrigues FG, da Silva G. Management of complex perineal fistula disease. Clin Colon Rectal Surg. 2016;29:92–100.
Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392.
Ritchie RD, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009;11:564–571.
van Koperen PJ, Safiruddin F, Bemelman WA, Slors JF. Outcome of surgical treatment for fistula in ano in Crohn’s disease. Br J Surg. 2009;96:675–679.
Present DH. Crohn’s fistula: current concepts in management. Gastroenterology. 2003;124:1629–1635.
Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41:77–86.
Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology. 1979;77:843–846.
Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95.
Hamamoto H, Gorman JH, Ryan LP, et al. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801.
Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379.
Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transpl. 2008;19:41–46.
Xie M, Qin H, Luo Q, et al. Comparison of adipose-derived and bone marrow mesenchymal stromal cells in a murine model of Crohn’s disease. Dig Dis Sci. 2017;62:115–123.
Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991.
Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (Nos. 81272656, 81572428, 81330014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Cao, Y., Ding, Z., Han, C. et al. Efficacy of Mesenchymal Stromal Cells for Fistula Treatment of Crohn’s Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 62, 851–860 (2017). https://doi.org/10.1007/s10620-017-4453-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4453-x