Abstract
Background
Prostaglandin E2 (PGE2) is the dominant prostaglandin in the colon and is associated with colonic inflammation. PGE2 levels are regulated not only by cyclooxygenases (COX-1 and COX-2) but also by 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the major PGE2-degrading enzyme. Information about the involvement of 15-PGDH in colonic inflammation is sparse.
Aim
We thus aimed to determine the gene expression and immunoreactivity (IR) of COX-1, COX-2, and 15-PGDH in colonic mucosa from patients with diverse inflammatory disorders: ulcerative colitis (UC), Crohn’s disease (CD), and acute diverticular disease (DD).
Methods
RNA from human colonic mucosa was extracted and assessed for gene expression by real-time PCR. Intact colon sections were processed for immunohistochemistry with immunostaining of the mucosal areas quantified using ImageJ.
Results
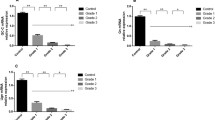
In colonic mucosa of both UC and CD, COX-2 mRNA and COX-2-IR were significantly increased, whereas 15-PGDH mRNA and 15-PGDH-IR were significantly reduced. In macroscopically undamaged acute DD mucosa, the opposite findings were seen: for both gene expression and immunoreactivity, there was a significant downregulation of COX-2 and upregulation of 15-PGDH. COX-1 mRNA and COX-1-IR remained unchanged in all diseases.
Conclusions
Our study for the first time demonstrated differential expression of the PGE2-related enzymes COX-2 and 15-PGDH in colonic mucosa from UC, CD, and acute DD. The reduction of 15-PGDH in IBD provides an additional mechanism for PGE2 increase in IBD. With respect to DD, alterations of PGE2-related enzymes suggest that a low PGE2 level may precede the onset of inflammation, thus providing new insight into the pathogenesis of DD.






Similar content being viewed by others
References
Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517.
Kaser A, Zeissig S, Blumberg RS. Genes and environment: how will our concepts on the pathophysiology of IBD develop in the future? Dig Dis Sci. 2010;28:395–405.
Kruis W, Jauch K-W, Kreis ME, Wexner SD. Diverticular Disease: Emerging Evidence in a Common Condition. Berlin: Springer; 2006.
Salzman H, Lillie D. Diverticular disease: diagnosis and treatment. Am Fam Phys. 2005;72:1229–1234.
Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182.
Miller JB, Jarosik C, Stanisic D, Wilson L Jr. Alterations in plasma and tissue prostaglandin levels in rabbits during luteal regression. Biol Reprod. 1983;29:824–832.
Burakoff R, Percy WH. Studies in vivo and in vitro on effects of PGE2 on colonic motility in rabbits. Am J Physiol. 1992;262:G23–G29.
Ding M, Kinoshita Y, Kishi K, et al. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins. 1997;53:199–216.
Takeuchi K, Yagi K, Kato S, Ukawa H. Roles of prostaglandin E-receptor subtypes in gastric and duodenal bicarbonate secretion in rats. Gastroenterology. 1997;113:1553–1559.
Tani S, Okuda M, Morishige R, Tanaka T. Gastric mucin secretion from cultured rat epithelial cells. Biol Pharm Bull. 1997;20:482–485.
White DM. Mechanism of prostaglandin E2-induced substance P release from cultured sensory neurons. Neuroscience. 1996;70:561–565.
Lou LH, Jing DD, Lai YX, et al. 15-PGDH is reduced and induces apoptosis and cell cycle arrest in gastric carcinoma. World J Gastroenterol. 2012;18:1028–1037.
Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med. 2014;6:233re2.
Dai L, Perera DS, King DW, et al. Hemokinin-1 stimulates prostaglandin E(2) production in human colon through activation of cyclooxygenase-2 and inhibition of 15-hydroxyprostaglandin dehydrogenase. J Pharmacol Exp Ther. 2011;340:27–36.
Otani T, Yamaguchi K, Scherl E, et al. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361–G368.
Hartig SM. Basic image analysis and manipulation in Image. J Curr Protoc Mol Biol. 2013;102:14.15.1–14.15.12.
Singer II, Kawka DW, Schloemann S, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306.
Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720.
Hendel J, Nielsen OH. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol. 1997;92:1170–1173.
Kang RY, Freire-Moar J, Sigal E, Chu CQ. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br J Rheumatol. 1996;35:711–718.
Roberts PJ, Morgan K, Miller R, Hunter JO, Middleton SJ. Neuronal COX-2 expression in human myenteric plexus in active inflammatory bowel disease. Gut. 2001;48:468–472.
Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD + -dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J Clin Endocrinol Metab. 1999;84:4645–4651.
Pecchi E, Priam S, Mladenovic Z, et al. A potential role of chondroitin sulfate on bone in osteoarthritis: inhibition of prostaglandin E(2) and matrix metalloproteinases synthesis in interleukin-1beta-stimulated osteoblasts. Osteoarthr Cartil. 2011;20:127–135.
Xun CQ, Tian ZG, Tai HH. Stimulation of synthesis de novo of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase in human promyelocytic leukaemia (HL-60) cells by phorbol ester. Biochem J. 1991;279:553–558.
Tong M, Tai HH. 15-Hydroxyprostaglandin dehydrogenase can be induced by dexamethasone and other glucocorticoids at the therapeutic level in A549 human lung adenocarcinoma cells. Arch Biochem Biophys. 2005;435:50–55.
Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev. 2011;30:409–417.
Mattii L, Ippolito C, Segnani C, et al. Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS ONE. 2013;8:e57023.
Bottner M, Barrenschee M, Hellwig I, et al. The GDNF system is altered in diverticular disease—implications for pathogenesis. PLoS ONE. 2013;8:e66290.
Liu L, Shang F, Markus I, Burcher E. Roles of substance P receptors in human colon circular muscle: alterations in diverticular disease. J Pharmacol Exp Ther. 2002;302:627–635.
Liu L, Markus I, Saghire HE, et al. Distinct differences in tachykinin gene expression in ulcerative colitis, Crohn’s disease and diverticular disease: a role for hemokinin-1? Neurogastroenterol Motil. 2011;23:e179–e480.
Tursi A, Brandimarte G, Elisei W, et al. Assessment and grading of mucosal inflammation in colonic diverticular disease. J Clin Gastroenterol. 2008;42:699–703.
Tursi A, Elisei W, Brandimarte G, et al. Mucosal expression of basic fibroblastic growth factor, Syndecan 1 and tumor necrosis factor-alpha in diverticular disease of the colon: a case-control study. Neurogastroenterol Motil. 2012;24:836–e936.
Tong M, Tai HH. Synergistic induction of the nicotinamide adenine dinucleotide-linked 15-hydroxyprostaglandin dehydrogenase by an androgen and interleukin-6 or forskolin in human prostate cancer cells. Endocrinology. 2004;145:2141–2147.
Nakada SY, Jerde TJ, Jacobson LM, et al. Cyclooxygenase-2 expression is up-regulated in obstructed human ureter. J Urol. 2002;168:1226–1229.
McKeown KJ, Challis JR. Regulation of 15-hydroxy prostaglandin dehydrogenase by corticotrophin-releasing hormone through a calcium-dependent pathway in human chorion trophoblast cells. J Clin Endocrinol Metab. 2003;88:1737–1741.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia (ID APP568861). We thank Fei Shang and the Histology and Microscopy Unit for technical assistance and Changfa Qu for specimen collection.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, L., King, D.W., Perera, D.S. et al. Inverse Expression of Prostaglandin E2-Related Enzymes Highlights Differences Between Diverticulitis and Inflammatory Bowel Disease. Dig Dis Sci 60, 1236–1246 (2015). https://doi.org/10.1007/s10620-014-3478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3478-7