Abstract
Background
Biliary atresia-induced cholestasis increases hepatic oxidative stress with eventual progression to cirrhosis and liver failure. Omega-3 fatty acids play a possible role in the regulation of oxidative stress and the improvement of cholestasis.
Aim
The goal of the present study is to investigate the role of dietary supplementation of fish omega-3 fatty acids in the reduction of hepatocellular damage by using a rat common bile duct ligation model.
Methods
Sprague–Dawley rats received either sham or bile duct ligation (BDL) and were divided into four study groups: Sham+saline (Sham+sal) group, Sham+Fish oil (Sham+FO) group, BDL+saline (BDL+sal) group, and BDL+Fish oil (BDL+FO) group. Rats from each group were assigned to receive, besides regular chow, once daily with either normal saline or fish omega-3 fatty acids (0.4 % of its own body weight) via gavage for 10 days. Samples of blood, liver tissue homogenates, and histological studies from different groups were analyzed at the end of the study.
Results
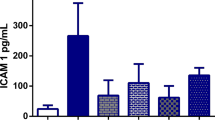
Rats from BDL+FO had significantly impaired liver function as compared to other study groups (p < 0.05 is of significant difference). Ishak scores and the TGF-b1 contents were significantly higher in rats that received BDL+FO, p < 0.05. Contrary to TGF-b1 liver content, rats from the BDL+FO group had the lowest glutathione levels among the study groups, p < 0.05.
Conclusions
Fish omega-3 fatty acids supplementation, albeit increased tissue content of DHA, tended to increase liver fibrosis in BDL rats, decrease liver glutathione level, and compromise hepatic function; fish oil supplementation to subjects with biliary atresia might be of potential hazard and should be used with caution.





Similar content being viewed by others
References
Alagille D. Extrahepatic biliary atresia. Hepatology. 1984;4:7S–10S.
Portincasa P, Grattagliano I, Testini M, et al. Parallel intestinal and liver injury during early cholestasis in the rat: modulation by bile salts and antioxidants. Free Radic Biol Med. 2007;42:1381–1391.
Sastre J, Serviddio G, Pereda J, et al. Mitochondrial function in liver disease. Front Biosci. 2007;12:1200–1209.
Huang LT, Tiao MM, Tain YL, Chen CC, Hsieh CS. Melatonin ameliorates bile duct ligation-induced systemic oxidative stress and spatial memory deficits in developing rats. Pediatr Res. 2009;65:176–180.
Yang H, Ramani K, Xia M, et al. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49:1982–1991.
Gautier M, Eliot N. Extrahepatic biliary atresia: morphological study of 98 biliary remnants. Arch Path Lab Med. 1981;105:397–402.
Chuang YH, Lan RY, Gershwin ME. The immunopathology of human biliary cell epithelium. Semin Immunopathol. 2009;31:323–331.
Needleman P, Raz A, Minkes MS, et al. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci USA. 1979;76(2):944–948.
Terano T, Salmon JA, Moncada S. Biosynthesis and biologic activity of leukotriene B5. Prostaglandins. 1984;27:217–232.
Katz DP, Schwartz S, Askanazi J. Biochemical and cellular basis for potential therapeutic value of n-3 fatty acids derived from fish oil. Nutrition. 1993;9:113.
Hu M-L, Frankel EN, Leibovitz BE, Tappel AL. Effect of dietary lipids and vitamin E on in vitro lipid peroxidation in rat liver and kidney homogenates. J Nutr. 1989;119:1574–1582.
Kaasgaard SG, Hølmer G, Høy CE, Behrens WA, Beare-Rogers JL. Effects of dietary linseed oil and marine oil on lipid peroxidation in monkey liver in vivo and in vitro. Lipids. 1992;27(10):740–745.
Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition. 2011;27(3):334–337.
Chen C-C, Chaung H-C, Chung M-Y, Huang L-T. Menhaden fish oil improves spatial memory in rat pups following recurrent pentylenetetrazole-induced seizures. Epilepsy Behav. 2006;8(3):516–521.
Chen C-C, Huang L-T, Tain Y-L, et al. Reduced brain content of arachidonic acid and docosahexaenoic acid is related to the severity of liver fibrosis. Dig Dis Sci. 2010;55:2831–2837.
Elizabeth M. Brunt, Grading and staging the histopathological lesion of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241–246.
Burke PA, Ling PR, Forse RA, Bistrian BR. Conditionally essential fatty acid deficiencies in end-stage liver disease. Nutrition. 1999;15:302.
Cabré E, Hernández-Pérez JM, Fluvià L, Pastor C, Corominas A, Gassull MA. Absorption and transport of dietary long-chain fatty acids in cirrhosis: a stable-isotope-tracing study. Am J Clin Nutr. 2005;81(3):692–701.
Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care. 2010;13:321–326.
Van Aerde JE, Duerksen DR, Gramlich L, et al. Intravenous fish oil emulsion attenuates total parenteral nutrition-induced cholestasis in newborn piglets. Pediatr Res. 1999;45:202–208.
Berard AM, Dumon MF, Darmon M. Dietary fish oil up-regulates cholesterol 7 alpha-hydroxylase mRNA in mouse liver leading to an increase in bile acid and cholesterol excretion. FEBS Lett. 2004;559:125–128.
Ramaprasad TR, Srinivasan K, Baskaran V, Sambaiah K, Lokesh BR. Spray-dried milk supplemented with alpha-linolenic acid or eicosapentaenoic acid and docosahexaenoic acid decreases HMG Co A reductase activity and increases biliary secretion of lipids in rats. Steroids. 2006;71:409–415.
Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41.
Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358.
Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765.
Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645.
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111.
Ono A, Utsugi M, Masubuchi K, et al. Glutathione redox regulates TGF-beta-induced fibrogenic effects through Smad3 activation. FEBS Lett. 2009;583:357–362.
Acknowledgments
This study was supported by a research grant (CMRP 890211) from the Chang-Gung Memorial Hospital to Dr. Chih-Cheng Chen. The authors also wish to thank Dr. Arthur Chen for his grammatical input and editing work on this paper.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chih-Cheng Chen and Chun-Yi Ho contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, CC., Ho, CY., Chaung, HC. et al. Fish Omega-3 Fatty Acids Induce Liver Fibrosis in the Treatment of Bile Duct-Ligated Rats. Dig Dis Sci 58, 440–447 (2013). https://doi.org/10.1007/s10620-012-2489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2489-5