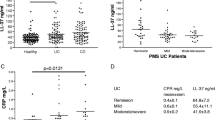
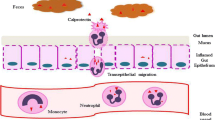
Abstract
Background
Since their discovery, S100 proteins have been associated with diverse diseases of inflammatory, degenerative, or malignant nature. Due to their participation in inflammation, they have also been studied with regard to inflammatory bowel disease (IBD).
Method
To provide a review of available literature, a PubMed, MEDLINE, and Embase-based literature search was performed, using all available nomenclature for each member of the S100 protein family, along with the terms inflammatory bowel disease, ulcerative colitis, Crohn’s disease, or indeterminate colitis.
Result
S100A8/A9, also known as calprotectin, S100A12, or calgranulin C and in a lesser extent S100P, are involved in the pathogenesis, activity, diagnosis, and therapeutic management of IBD. The majority of available literature is focused primarily on S100A8/9, although there is growing evidence on the significance of S100A12. Most studies emphasize the potential merit of S100A8/A9 and S100A12, as markers for differential diagnosis, monitoring of activity, or disease relapse, in IBD. Limitations, regarding the diagnostic utility of these markers, seem to exist and are mainly related to the publication of conflicting results, i.e., for IBD activity, and to the fact that S100A8/A9 and S100A12 are not disease-specific.
Conclusions
Although the existing data link specific S100 proteins with IBD, there are still several drawbacks in the use of these markers for diagnostic purposes. Thus, it seems that further research is mandatory in order to eliminate the impact of confounding factors but also to detect additional associations between S100 proteins and IBD or novel S100 proteins with a closer correlation with IBD.


Similar content being viewed by others
References
Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commmun. 1965;19:739–744.
Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214.
Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551.
Foell D, Frosch M, Sorg C, et al. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:3751.
Foell D, Wittkowski H, Vogl T, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37.
Leclerc E, Fritz G, Weibel M, et al. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J Biol Chem. 2007;282:31317–31331.
Salama I, Malone PS, Mihaimeed F, et al. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364.
Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85:1373–1380.
Pleger ST, Most P, Boucher M, et al. Stable myocardial-specific AAV6–S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515.
Eckert RL, Broome AM, Ruse M, et al. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33.
Giovannoni G. Multiple sclerosis cerebrospinal fluid biomarkers. Dis Markers. 2006;22:187–196.
Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–3058.
Hancq S, Salmon I, Brotchi J, et al. S100A5: a marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol. 2004;30:178–187.
Pierce A, Barron N, Linehan R, et al. Identification of a novel, functional role for S100A13 in invasive lung cancer cell lines. Eur J Cancer. 2008;44:151–159.
Landriscina M, Schinzari G, Di Leonardo G, et al. S100A13, a new marker of angiogenesis in human astrocytic gliomas. J Neurooncol. 2006;80:251–259.
Michetti F, Gazzolo D. S100B testing in pregnancy. Clin Chim Acta. 2003;335:1–7.
Esposito G, Cirillo C, Sarnelli G, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–925.
Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10:445–456.
Roth J, Vogl T, Sorg C, et al. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158.
Vogl T, Propper C, Hartmann M, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296.
El-Rifai W, Moskaluk CA, Abdrabbo MK, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826.
Liu J, Li X, Dong GL, et al. In silico analysis and verification of S100 gene expression in gastric cancer. BMC Cancer. 2008;8:261.
Sapkota D, Bruland O, Bøe OE, et al. Expression profile of the S100 gene family members in oral squamous cell carcinomas. J Oral Pathol Med. 2008;37:607–615.
Ji J, Zhao L, Wang X, et al. Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:480–486.
Rodriguez JA, Li M, Yao Q, et al. Gene overexpression in pancreatic adenocarcinoma: diagnostic and therapeutic implications. World J Surg. 2005;29:297–305.
Capella C, Riva C, Rindi G, et al. Endocrine tumors of the duodenum and upper jejunum. A study of 33 cases with clinico-pathological characteristics and hormone content. Hepatogastroenterology. 1990;37:247–252.
Srivastava MD, Kulaylat MN. Gene expression profiles of late colonic Crohn’s disease. J Med. 2004;35:233–255.
Rugtveit J, Nilsen EM, Bakka A, et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493–1505.
Sarsfield P, Jones DB, Wright DH. Accessory cells in Crohn’s disease of the terminal ileum. Histopathology. 1996;28:213–219.
Waraich T, Sarsfield P, Wright DH. The accessory cell populations in ulcerative colitis: a comparison between the colon and appendix in colitis and acute appendicitis. Hum Pathol. 1997;28:297–303.
Verstege MI, ten Kate FJ, Reinartz SM, et al. Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn’s disease. J Histochem Cytochem. 2008;56:233–241.
Kubota Y, Petras RE, Ottaway CA, et al. Colonic vasoactive intestinal peptide nerves in inflammatory bowel disease. Gastroenterology. 1992;102:1242–1251.
Foell D, Kucharzik T, Kraft M, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853.
Leach ST, Yang Z, Messina I, et al. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321–1331.
Rugtveit J, Haraldsen G, Hogasen AK, et al. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14 + L1 + monocyte derived cells. Gut. 1995;37:367–373.
Lügering N, Stoll R, Kucharzik T, et al. Immunohistochemical distribution and serum levels of the Ca(2 +)-binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn’s disease. Digestion. 1995;56:406–414.
Kapsoritakis AN, Georgoulias PA, Manolakis AC, et al. Serum S100A12, a marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2008;57:A138.
de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn’s disease. Inflamm Bowel Dis. 2006;12:566–572.
Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–1713.
Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008;14:359–366.
Foell D, Wittkowski H, Ren Z, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–192.
Karl J, Wild N, Tacke M, et al. Improved diagnosis of colorectal cancer using a combination of fecal occult blood and novel fecal protein markers. Clin Gastroenterol Hepatol. 2008;6:1122–1128.
Fagerberg UL, Lööf L, Lindholm J, et al. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414–420.
von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813.
Joishy M, Davies I, Ahmed M, et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48–54.
D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437.
Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169.
Dolwani S, Metzner M, Wassell JJ, et al. Diagnostic accuracy of faecal calprotectin estimation in prediction of abnormal small bowel radiology. Aliment Pharmacol Ther. 2004;20:615–621.
Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–39.
Otten CM, Kok L, Witteman BJ, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280.
Limburg PJ, Ahlquist DA, Sanborn WJ, et al. Faecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–2837.
Caroccio A, Iacono G, Cottone M, et al. Diagnostic accuracy of faecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861–867.
Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liv Dis. 2003;35:642–647.
Leach ST, Mitchell HM, Geczy CL, et al. S100 calgranulin proteins S100A8, S100A9 and S100A12 are expressed in the inflamed gastric mucosa of Helicobacter pylori-infected children. Can J Gastroenterol. 2008;22:461–464.
Tursi A, Brandimarte G, Elisei W, et al. Faecal calprotectin in colonic diverticular disease: a case-control study. Int J Colorectal Dis. 2009;24:49–55.
Bremner A, Roked S, Robinson R, et al. Faecal calprotectin in children with chronic gastrointestinal symptoms. Acta Paediatr. 2005;94:1855–1858.
Tibble J, Sigthorsson G, Foster R, et al. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402–408.
Hoff G, Grotmol T, Thiis-Evensen E, et al. Testing for faecal calprotectin (PhiCal) in the Norwegian Colorectal Cancer Prevention trial on flexible sigmoidoscopy screening: comparison with an immunochemical test for occult blood (FlexSure OBT). Gut. 2004;53:1329–1333.
Wedlake L, McGough C, Hackett C, et al. Can biological markers act as non-invasive, sensitive indicators of radiation-induced effects in the gastrointestinal mucosa? Aliment Pharmacol Ther. 2008;27:980–987.
Reinders CA, Jonkers D, Janson EA, et al. Rectal nitric oxide and fecal calprotectin in inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1151–1157.
Poullis A, Foster R, Shetty A, et al. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279–284.
Langhorst J, Elsenbruch S, Mueller T, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–1091.
Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46.
Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398.
Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229.
Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553.
Vieira A, Fang CB, Rolim EG, et al. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221.
Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leucocytes. Inflamm Bowel Dis. 2009;15:1851–1858.
Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leucocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169.
Kolho KL, Raivio T, Lindahl H, et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720–725.
Scarpa M, D’Incà R, Basso D, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861–869.
Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22.
Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368.
D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014.
Gisbert JP, Bermejo F, Perez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198.
Orlando A, Modesto I, Castiglione F, et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17–22.
Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663–674.
Thomas P, Rihani H, Røseth A, et al. Assessment of ileal pouch inflammation by single-stool calprotectin assay. Dis Colon Rectum. 2000;43:214–220.
Johnson MW, Maestranzi S, Duffy AM, et al. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol. 2008;20:174–179.
Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901.
Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043.
Ghavami S, Kerkhoff C, Los M, et al. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–175.
Ghavami S, Kerkhoff C, Chazin WJ, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008;1783:297–311.
Nakayama Y, Inoue Y, Minagawa N, et al. Relationships between S-100 protein-positive cells and clinicopathological factors in patients with colorectal cancer. Anticancer Res. 2003;23:4423–4426.
Nagorsen D, Voigt S, Berg E, et al. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62.
Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818.
Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–798.
Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177.
Xiang JY, Ouyang Q, Li GD, et al. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57.
Ho GT, Lee HM, Brydon G, et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–678.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manolakis, A.C., Kapsoritakis, A.N., Tiaka, E.K. et al. Calprotectin, Calgranulin C, and Other Members of the S100 Protein Family in Inflammatory Bowel Disease. Dig Dis Sci 56, 1601–1611 (2011). https://doi.org/10.1007/s10620-010-1494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1494-9