Abstract
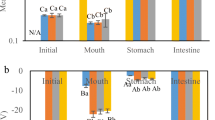
The aim of this study was to design food grade matrices to deliver microencapsulated fish oil to the large bowel of the rat where the potential exists to retard inflammation and cancer development. Digestion in simulated gastric fluid and intestinal fluid demonstrated that only 4–6% of oil was released from the following dried emulsion formulations: 50% fish oil encapsulated in heated casein-glucose-dried glucose syrup (1:1:1) (Cas-Glu-DGS-50); 25% fish oil in casein-modified resistant starch (Hylon VII) (1:1) (Cas-Hylon-25); or 25% fish oil in Cas-Glu-Hylon (1:1:1) (Cas-Glu-Hylon-25). A short-term gavage study (0–12 h) with fish oil and Cas-Glu-DGS-50 demonstrated the appearance of fish oil long chain (LC) n-3 polyunsaturated fatty acids (PUFA) into the plasma indicating specific small intestinal absorption with little LC n-3 PUFA reaching the large bowel. In a 2-week-long term, daily gavage study, the bioavailability of fish oil and fish oil in Cas-Glu-DGS-50 or Cas-Hylon-25 demonstrated that fish oil and Cas-Glu-DGS-50 LC n-3 PUFA were incorporated into the tissue of the small intestine and colon, whereas Cas-Hylon-25 was resistant to degradation in the small intestine. The use of modified Hylon VII for targeted colonic delivery was confirmed in the final short-term gavage study (0–14 h) using Cas-Glu-Hylon-25 with [14C]-trilinolenin as a marker incorporated into the microcapsules, where up to 60% of the labeled oil reached the large bowel. Depending on the microencapsulating matrix employed, fish oil can be delivered selectively to the small intestine or to a high degree to the large bowel.






Similar content being viewed by others
References
Pattison DJ, Symmons DP, Young A (2004) Does diet have a role in the aetiology of rheumatoid arthritis? Proc Nutr Soc 63:137–143. doi:10.1079/PNS2003319
MacLean CH, Mojica WA, Newberry SJ, Pencharz J, Garland RH, Tu W et al (2005) Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr 82:611–619
De Lorgeril M (2007) Essential polyunsaturated fatty acids, inflammation, atherosclerosis and cardiovascular diseases. Subcell Biochem 42:283–297
Akedo I, Ishikawa H, Nakamura T, Kimura K, Ikuko T, Suzuki T et al (1998) Three cases with familial adenomatous polyposis diagnosed as having malignant lesions in the time course of a long-term trial using docosahexaenoic acid (DHA)-concentrated fish oil capsules. Jpn J Clin Oncol 28:762–765. doi:10.1093/jjco/28.12.762
Huang YC, Jessup JM, Forse RA, Flickner S, Pleskpw D, Anastopoulos HT et al (1996) N-3 fatty acids decrease colonic epithelial cell proliferation in at risk bowel mucosa. Lipids 31:S313–S317. doi:10.1007/BF02637099
Nkondlock A, Ghadirian P (2004) Dietary carotenoids and risk of colon cancer: case-control study. Int J Cancer 110:110–116. doi:10.1002/ijc.20066
Raju J, McCarthy B, Bird RP (2002) Steady state levels of transforming growth factor-beta1 and -beta2 mRNA and protein expression are elevated in colonic tumors in vivo irrespective of dietary lipids intervention. Int J Cancer 100:635–641. doi:10.1002/ijc.10522
Rose DP, Connolly JM (1999) Omega-3 fatty acids as cancer chemoprotective agents. Pharmacol Ther 83:217–244. doi:10.1016/S0163-7258(99)00026-1
Roynette CE, Calder PC, Dupertuis YM, Pichard C (2004) n-3 Polyunsaturated fatty acids and colon cancer prevention. Clin Nutr 23:139–151. doi:10.1016/j.clnu.2003.07.005
Dommels YEM, Haring MMG, Keestra NGM, Alink GM, van Bladeren PJ, van Ommen B (2003) The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE2 synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis 24:385–392. doi:10.1093/carcin/24.3.385
Jordan A, Stein J (2003) Effect of an omega-3 fatty acid containing lipid emulsion alone and in combination with 5-fluorouracil (5-FU) on growth of colon cancer cell line Caco-2. Eur J Nutr 42:324–331. doi:10.1007/s00394-003-0427-1
Danbara N, Yuri T, Tsujita-Kyutoku M, Sato M, Senzaki H, Takada H et al (2004) Conjugated docosahexaenoic acid is a potent inducer of cell cycle arrest and apoptosis and inhibits growth of colon 201 human colon cancer cells. Nutr Cancer 50:71–79. doi:10.1207/s15327914nc5001_10
Hofmanova J, Vaculova A, Kozubik A (2005) Polyunsaturated fatty acids sensitize human colon adenocarcinoma HT-29 cells to death receptor mediated apoptosis. Cancer Lett 218:33–41. doi:10.1016/j.canlet.2004.07.038
Hofmanova J, Vaculova A, Lojek A, Kozubik A (2005) Interaction of polyunsaturated fatty acids and sodium butyrate during apoptosis in human HT-29 human colon adenocarcinoma cells. Eur J Nutr 44:40–51. doi:10.1007/s00394-004-0490-2
Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M (1996) Effect of enteric-coated fish-oil preparation on relapses in Crohn’s disease. N Engl J Med 334:1557–1560. doi:10.1056/NEJM199606133342401
Calviello G, Serini S, Piccione E (2007) N-3 polyunsaturated fatty acids and prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem 14:3059–3069. doi:10.2174/092986707782793934
Lupton JR (2004) Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr 134:479–482
von Engelhardt W, Bartels J, Kirschberger S, Meyer zu Düttingdorf HD, Busche R (1998) Role of short-chain fatty acids in the hind gut. Vet Q 20(suppl 3):S52–S59
Le Leu R, Hu Y, Young GP (2002) Effects of resistant starch and non-starch polysaccharides on colonic luminal environment and genotoxin-induced apoptosis in the rat. Carcinogenesis 23:713–719. doi:10.1093/carcin/23.5.713
Sanguansri L, Augustin MA (2006) Microencapsulation and delivery of omega-3 fatty acids. In: Shi J (ed) Functional food engineering technologies and processing. CRC Press, NY, pp 297–327
Champagne CP, Fustier P (2007) Microencapsulation for improved deliver of bioactive compounds into foods. Curr Opin Biotechnol 18:184–190. doi:10.1016/j.copbio.2007.03.001
Wallace JMW, McCabe AJ, Robson PJ, Keogh MK, Murray CA, Kelly PM et al (2000) Bioavailability of n-3 polyunsaturated fatty acids (PUFA) in foods enriched with microencapsulated fish oil. Ann Nutr Metab 44:157–162. doi:10.1159/000012839
Lemprecht A, Schafer U, Lehr CM (2001) Influences of process parameters on preparation of micro particle used as a carrier system for Ω-3 unsaturated fatty acid ethyl esters used in supplementary nutrition. J Microencapsul 18:347–357. doi:10.1080/02652040010000433
Kolanowski W, Laufenberg G, Kunz B (2004) Fish oil stabilisation by microencapsulation with modified cellulose. Int J Food Sci Nutr 55:333–343. doi:10.1080/09637480410001725157
Tozaki H, Komoike J, Tada C, Maruyama T, Terabe T, Suzuki T et al (1997) Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci 86:1016–1021. doi:10.1021/js970018g
Morishita M, Kajita M, Suzuki A, Takayama K, Chiba Y, Tokiwa S et al (2000) The dose-related hypoglycaemic effects of insulin emulsions incorporating highly purified EPA and DHA. Int J Pharm 201:175–185. doi:10.1016/S0378-5173(00)00411-7
Lorenzo-Lamosa ML, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1998) Design of microencapsulated chitosan microspheres for colonic delivery. J Control Release 52:109–118. doi:10.1016/S0168-3659(97)00203-4
Augustin MA, Sanguansri P, Htoon A (2008) Functional performance of a resistant starch ingredient modified using a microfluidiser. Innov Food Sci Emerg Tech 9:224–231. doi:10.1016/j.ifset.2007.11.003
Pharmacopeia US (2000) National formulatory (USP 24 NF 19). Rockville, MD
Stahl GE, Fayer JC, Ling SC, Watkins JB (1991) Comparison of nonabsorbable markers Poly R-478 and [14C] PEG-4000 for use in developmental absorption studies. J Pediatr Gastroenterol Nutr 12:485–493. doi:10.1097/00005176-199105000-00013
Tuleu C, Andrieux C, Cherbuy C, Darcy-Vrillon B, Duee PH, Chaumeil JC (2001) Colonic delivery of sodium butyrate via oral route: acrylic coating design of pellets and in vivo evaluation in rats. Methods Find Exp Clin Pharmacol 23:245–253. doi:10.1358/mf.2001.23.5.662119
Nilsson A, Hjelte L, Strandvik B (1992) Incorporation of dietary [14C]arachidonic acid and [3H]eicosapentaenoic acid into tissue lipids during absorption of a fish oil emulsion. J Lipid Res 33:1295–1305
Nilsson A, Hjelte L, Strandvik B (1996) Metabolism of orally fed [3H]-eicosapentaenoic acid and [14C]-arachidonic acid in essential fatty acid-deficient rats. Scand J Clin Lab Invest 56:219–227. doi:10.3109/00365519609088611
Patten GS, Bird AR, Topping DL, Abeywardena MY (2004) Effects of convenience rice congee supplemented diets on guinea pig whole animal and gut growth, caecal digesta SCFA and in vitro contractility. Asia Pac J Clin Nutr 13:92–100
Patten GS, Conlon MA, Bird AR, Adams MJ, Topping DL, Abeywardena MY (2006) Interactive effects of dietary resistant starch and fish oil on short-chain fatty acid production and agonist-induced contractility in ileum of young rats. Dig Dis Sci 51:254–261. doi:10.1007/s10620-006-3121-3
Park HS, Lee JY, Cho SH, Baek HJ, Lee SJ (2002) Colon delivery of prednisolone based on chitosan coated polysaccharide tablets. Arch Pharm Res 25:964–968
Shinohara H, Williams C, Koldovsky O (1995) The use of Poly R-478 as a marker to determine gastric emptying and intestinal propulsive motility in suckling rats. Physiol Res 44:281–286
Hussein Z, Friedman M (1990) Release and absorption characteristics of novel theophylline sustained-release formulations: in vitro-in vivo correlation. Pharm Res 7:1167–1171. doi:10.1023/A:1015988410977
Aiedah K, Taha MO (1999) Synthesis of chitosan succinate and chitosan phthalate and their evaluation as suggested matrices in orally administered, colon-specific drug delivery systems. Arch Pharm 332:103–107. doi:10.1002/(SICI)1521-4184(19993)332:3≤103::AID-ARDP103≥3.0.CO;2-U
Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL (2004) Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids 39:955–961. doi:10.1007/s11745-004-1317-0
Patten GS, Adams MJ, Dallimore JA, Abeywardena MY (2005) Dietary fish oil dose-response effects on ileal phospholipid fatty acids and contractility. Lipids 40:925–929. doi:10.1007/s11745-005-1453-6
Berk PD, Stump DD (1999) Mechanisms of cellular uptake of long chain free fatty acids. Mol Cell Biochem 192:17–31. doi:10.1023/A:1006832001033
Bradbury MW, Berk PD (2004) Lipid metabolism in hepatic steatosis. Clin Liver Dis 8:639–671. doi:10.1016/j.cld.2004.04.005
Bretillon L, Chardigny JM, Sebedio JL, Poullain D, Noel JP, Vatele JM (1998) Oxidative metabolism of (1–14C) mono-trans isomers of linoleic and α-linolenic acids in the rat. Biochim Biophys Acta 1390:207–214
Cunnane SC (2001) Application of new methods and analytical approaches to research on polyunsaturated fatty acid homeostasis. Lipids 36:975–979. doi:10.1007/s11745-001-0808-3
Lenaerts V, Moussa I, Dumoulin Y, Mebsout F, Chouinard F, Szabo P et al (1998) Cross-linked high amylose starch for controlled release of drugs: recent advances. J Control Release 53:225–234. doi:10.1016/S0168-3659(97)00256-3
Chourasia MK, Jain SK (2004) Polysaccharides for colon targeted drug delivery. Drug Deliv 11:129–148. doi:10.1080/10717540490280778
Bird AR, Vuaran MS, King RA, Noakes M, Keough J, Morell MK et al (2007) Wholegrain foods from novel high amylose barley variety (Himalaya 292) improve indices of bowel health in human subjects. Br J Nutr 99:1032–1040
Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G et al (1998) Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol 275:G1414–G1422
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Kossena GA, Charman WN, Boyd BJ, Dunstan DE, Porter CJ (2004) Probing drug solubilisation patterns in the gastrointestinal tracts after administration of lipid-based delivery systems: a phase diagram approach. J Pharm Sci 93:332–338. doi:10.1002/jps.10554
Fujita F, Matsuoka H, Hirooka K (2007) Regulation of fatty acid metabolism in bacteria. Mol Microbiol 66:829–839. doi:10.1111/j.1365-2958.2007.05947.x
Jorgensen JR, Fitch MD, Mortensen PB, Fleming SE (2002) Absorption and metabolism of octanoate by the rat colon in vivo: concentration dependency and influence of alternate fuels. Gut 51:76–81. doi:10.1136/gut.51.1.76
Calderaro V, De Simone B, Giovane A, Quaggliuolo L, Servillo L, Giordano C et al (1991) Mechanism of arachidonic acid transport across rabbit distal colonic mucosa. Am J Physiol 261:G451–G457
Chen ZY, Itsfan NW (2000) Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids 63:301–308. doi:10.1054/plef.2000.0218
Narayanan BA, Narayanan NK, Reddy BS (2001) Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol 19:1255–1262
Murray NR, Weems C, Chen L, Leon J, Yu W, Davidson LA et al (2002) Protein kinase C betaII and TGFbetaRII in omega-3 fatty acid mediated inhibition of colon carcinogenesis. J Cell Biol 157:915–920. doi:10.1083/jcb.200201127
Calviello G, Resci F, Serini S, Piccioni E, Toesca A, Boninsegna A et al (2007) Docosahexaenoic acid induces proteosome-dependent degradation of β-catenin, down-regulation of surviving and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis 28:1202–1209. doi:10.1093/carcin/bgl254
Camuesco D, Comalada D, Concha A, Nieto A, Sierra S, Xaus J et al (2006) Intestinal anti-inflammatory activity of combined quercetrin and dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, in rats with DSS-induced colitis. Clin Nutr 25:466–476. doi:10.1016/j.clnu.2005.12.009
Turner D, Zlotkin SH, Shah PS, Griffiths AM (2007) Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 18(2):CD006320
Acknowledgments
The authors thank Michael Adams for animal husbandry and fatty acid analysis and Li Jiang Cheng, Rangika Weerakkody and Zhiping Shen for fatty acid analysis and in vitro oil release studies. Thanks also to Lynne Cobiac, Trevor Lockett and David Topping for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10620-011-1618-x
Rights and permissions
About this article
Cite this article
Patten, G.S., Augustin, M.A., Sanguansri, L. et al. Site Specific Delivery of Microencapsulated Fish Oil to the Gastrointestinal Tract of the Rat. Dig Dis Sci 54, 511–521 (2009). https://doi.org/10.1007/s10620-008-0379-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0379-7