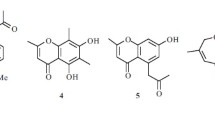
Two new coumarins, 6-hydroxymethyl-3-prenyl-7-methoxycoumarin (1) and 6-hydroxyethyl-3-prenyl-7- methoxycoumarin (2), together with three known coumarins (3–5), were isolated from the roots and stems of Yunyan-300, a variety of Nicotiana tabacum L. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were tested for their anti-methicillinresistant Staphylococcus aureus (anti-MRSA) activity and their antioxidant activity. The results revealed that compounds 1 and 2 showed good anti-MRSA inhibitions with IZD of 12.2 ± 1.8 and 11.8 ± 1.5 mm, respectively, and antioxidant activity with an IC50 value of 4.65 and 4.85 μg/mL, respectively.

Similar content being viewed by others
References
A. Rodgman and T. A. Perfetti, The Chemical Components of Tobacco and Tobacco Smoke, CRC Press Taylor and Francis Group, Boca Raton, Florida, 2008.
The Editorial Committee of the Administration Bureau of Flora of China, Flora of China, Vol. 67, Beijing Science and Technology Press, Beijing, 2005.
R. L. Stedman, Chem. Rev., 68, 153 (1968).
C. Y. Yang, Y. Lin, H. X. Yuan, W. P. Yang, X. Wei, and Z. L. Huang, Fitoterapia, 128, 242 (2018).
J. K. Sui, C. K. Wang, X. F. Liu, N. Fang, Y. H. Liu, W. J. Wang, N. Yan, H. B. Zhang, Y. M. Du, and X. M. Liu, Molecules, 23, 2511 (2018).
S. Z. Shang, W. Zhao, J. G. Tang, X. M. Xu, H. D. Sun, J. X. Pu, Z. H. Liu, M. M. Miao, Y. K. Chen, and G. Y. Yang, Fitoterapia, 108, 1 (2016).
P. S. Yang, S. Y. Tang, C. B. Liu, L. Ye, F. M. Zhang, P. He, Z. H. Liu, Y. K. Chen, M. M. Miao, Q. P. Shen, and J. Q. Wang, J. Asian Nat. Prod. Res., 21, 109 (2019).
S. Z. Shang, W. Zhao, J. G. Tang, J. X. Pu, D. L. Zhu, L. Yang, H. D. Sun, G. Y. Yang, and Y. K. Chen, Phytochem. Lett., 17, 173 (2016).
M. M. Miao, L. Li, Q. P. Shen, C. B. Liu, Y. K. Li, T. Zhang, F. M. Zhang, P. He, K. M. Wang, R. Z. Zhu, Y. K. Chen, and G. Y. Yang, Fitoterapia, 103, 260 (2015).
S. Z. Shang, J. L. Shi, J. G. Tang, J. X. Jiang, W. Zhao, X. D. Zheng, P. Lei, J. M. Han, C. Y. Wang, D. L. Yuan, G. Y. Yang, Y. K. Chen, and M. M. Miao, Nat. Prod. Res., 33, 157 (2019).
S. K. Chien, L. C. Chen, H. C. Huang, L. C. Chen, J. W. Hsiao, M. J. Cheng, and J. J. Chen, Chem. Nat. Compd., 54, 1044 (2018).
H. Tokumoto, H. Shimomura, T. Hakamatsuka, Y. Ozeki, and Y. Goda, Biol. Pharm. Bull., 39, 1263 (2016).
B. Sun, Y. X. Tian, F. Zhang, Q. Chen, Y. Zhang, Y. Luo, X. R. Wang, F. C. Lin, J. Yang, and H. R. Tang, Biomolecules, 8, 114 (2018).
S. Z. Shang, Y. X. Duan, X. Zhang, J. X. Pu, H. D. Sun, Z. Y. Chen, M. M. Miao, G. Y. Yang, and Y. K. Chen, Phytochem. Lett., 7, 413 (2014).
C. B. Liu, Q. P. Shen, Y. Wang, F. M. Zhang, P. He, X. X. Si, K. M. Wang, R. Z. Zhu, N. J. Xiang, and Z. H. Liu, Chem. Nat. Compd., 52, 992 (2016).
C. Lei, W. X. Xu, J. Wu, S. J. Wang, J. Q. Sun, Z. Y. Chen, and G. Y. Yang, Chem. Nat. Compd., 51, 43 (2015).
A. V. Buntic, O. S. Stajkovic-Srbinovic, D. I. Delic, S. I. Dimitrijevic-Brankovic, and M. D. Milic, J. Serb. Chem. Soc., 84, 129 (2019).
M. I. Hussain, Q. A. Syed, M. N. K. Khattak, B. Hafez, M. J. Reigosa, and A. El-Keblawy, Biologia, 74, 863 (2019).
A. Ibrar, S. A. Shehzadi, F. Saeed, and I. Khan, Bioorg. Med. Chem., 26, 3731 (2018).
S. Allison, S. J. Burks, and R. T. Taylor, Tetrahedron, 23, 9737 (1991).
S. Lee, D. S. Shin, J. S. Kim, K. B. Oh, and S. S. Kang, Arch. Pharm. Res., 26, 449 (2003).
D. Q. Yu and J. S. Yang, Handbook of Analytical Chemistry, Vol. 7, Nuclear Magnetic Resonance Spectroscopy, Chemical Industry Press, 2nd Ed, Beijing, 1999.
B. A. Burke and H. Parkins, Phytochemistry, 18, 1073 (1979).
Q. F. Hu, Y. L. Meng, X. D. Rui, Y. H. Qin, Z. Y. Yang, G. L. Zhao, Z. X. Yang, X. M. Gao, and T. F. Li, Chem. Nat. Compd., 50, 994 (2014).
W. S. Kong, H. H. Xing, J. Li, L. Ye, X. Liu, Y. P. Li, G. X. Rao, M. Zhou, G. Y. Yang, Q. F. Hu, Y. K. Li, and X. M. Li, Chem. Nat. Compd., 54, 1048 (2018).
E. Ozkan, F. P. Karakas, A. B. Yildirim, I. Tas, I. Eker, M. Z. Yavuz, and A. U. Turker, Prog. Nutr., 21, 652 (2019).
M. B. Pisano, A. Kumar, R. Medda, G. Gatto, R. Pal, A. Fais, B. Era, S. Cosentino, E. Uriarte, L. Santana, F. Pintus, and M. J. Matos, Molecules, 24, 2815 (2019).
Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, Vol. 32, Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 9th edition, 2012.
E. Tripoli, M. Guardia, S. Giammanco, D. Majo, and M. Giammanco, Food Chem., 104, 466 (2007).
Acknowledgment
This project was supported financially by the Foundation of Yunnan Tobacco Industry Co. Ltd. (2019JC04 and 2018JC07), the Foundation of China Tobacco Company (No. 110201901016), the high-tech projects of Yunnan Province (2017ZE027), and the National Natural Science Foundation of China (No. 31860100).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2020, pp. 693–696.
Rights and permissions
About this article
Cite this article
Zhu, LJ., Luo, D., Lv, N. et al. Two New Coumarins from the Roots and Stems of Nicotiana tabacum and their Bioactivity. Chem Nat Compd 56, 806–810 (2020). https://doi.org/10.1007/s10600-020-03157-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03157-1