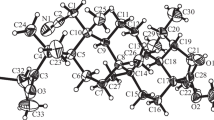
A macrocyclic glycoterpenoid containing the diterpenoid dihydroisosteviol (16-hydroxy-ent-beyeran-19- oic acid) and α,α′-trehalose linked by an ester spacer was synthesized.
Similar content being viewed by others
References
B. F. Garifullin, O. V. Andreeva, I. Yu. Strobykina, V. M. Babaev, and V. E. Kataev, Macroheterocycles, 6, 184 (2013).
O. V. Andreeva, I. Yu. Strobykina, O. N. Kataeva, O. B. Dobrynin, V. M. Babaev, I. Kh. Rizvanov, D. R. Islamov, and V. E. Kataev, Macroheterocycles, 6, 315 (2013).
R. R. Sharipova, B. F. Garifullin, O. V. Andreeva, I. Yu. Strobykina, O. B. Bazanova, and V. E. Kataev, Zh. Org. Khim., 51, 437 (2015); R. R. Sharipova, B. F. Garifullin, O. V. Andreeva, I. Yu. Strobykina, O. B. Bazanova, and V. E. Kataev, Russ. J. Org. Chem., 51, 424 (2015).
O. V. Andreeva, R. R. Sharipova, B. F. Garifullin, I. Yu. Strobykina, and V. E. Kataev, Chem. Nat. Compd., 51, 689 (2015).
M. Gavagnin, M. Carbone, P. Amodeo, E. Mollo, R. M. Vitale, V. Roussis, and G. Cimino, J. Org. Chem., 72, 5625 (2007).
V. A. Al_fonsov, G. A. Bakaleinik, A. T. Gubaidullin, V. E. Kataev, G. I. Kovylyaeva, A. I. Konovalov, I. A. Litvinov, I. Yu. Strobykina, S. I. Strobykin, O. V. Andreeva, and M. G. Korochkina, Zh. Obshch. Khim., 70, 1018 (2000); V. A. Alfonsov, G. A. Bakaleinik, A. T. Gubaidullin, V. E. Kataev, G. I. Kovylyaeva, A. I. Konovalov, I. A. Litvinov, I. Yu. Strobykina, S. I. Strobykin, O. V. Andreeva, and M. G. Korochkina, Russ. J. Gen. Chem., 70, 1318 (2000).
V. E. Kataev, O. I. Militsina, I. Yu. Strobykina, G. I. Kovylyaeva, R. Z. Musin, O. V. Fedorova, G. L. Rusinov, M. N. Zueva, G. G. Mordovskoi, and A. G. Tolstikov, Khim.-farm. Zh., 40 (9), 12 (2006); V. E. Kataev, O. I. Militsina, I. Yu. Strobykina, G. I. Kovylyaeva, R. Z. Musin, O. V. Fedorova, G. L. Rusinov, M. N. Zueva, G. G. Mordovskoi, and A. G. Tolstikov, Pharm. Chem. J., 40 (9), 473 (2006).
B. P. Mundy, M. G. Ellerd, and F. G. Favaloro, Name Reactions and Reagents in Organic Synthesis, John Wiley & Sons, New Jersey, 2005, pp. 352, 793.
B. C. Holland and N. W. Gilman, Synth. Commun., 4, 203 (1974).
T. C. Baddeley and J. L. Wardell, J. Carbohydr. Chem., 28, 198 (2009).
M. Carbone, M. Gavagnin, E. Mollo, M. Bidello, V. Roussis, and G. Cimino, Tetrahedron, 64, 191 (2008).
R. N. Khaibullin, I. Yu. Strobykina, V. E. Kataev, O. A. Lodochnikova, A. T. Gubaidullin, and R. Z. Musin, Zh. Obshch. Khim., 79, 795 (2009); R. N. Khaibullin, I. Yu. Strobykina, V. E. Kataev, O. A. Lodochnikova, A. T. Gubaidullin, and R. Z. Musin, Russ. J. Gen. Chem., 79, 967 (2009).
E. Mosettig and W. R. Nes, J. Org. Chem., 20, 884 (1955).
H. Bredereck, Chem. Ber., 63, 959 (1930).
Acknowledgment
The work was sponsored by the Russian Science Foundation (Grant No. 14-50-00014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ttranslated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2015, pp. 760–763.
Rights and permissions
About this article
Cite this article
Garifullin, B.F., Sharipova, R.R., Strobykina, I.Y. et al. Synthesis of the First Macrocyclic Glycoterpenoid Based on Trehalose and the Diterpenoid Isosteviol. Chem Nat Compd 51, 886–889 (2015). https://doi.org/10.1007/s10600-015-1440-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1440-3