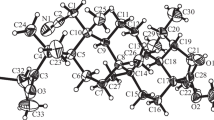
Acid-catalyzed formation of the peroxyacetal of cyclohexanone bis-hydroperoxide and a triterpene ketone synthesized for the first time 19β,28-epoxy-28-oxo-18α-olean-3-spiro-6′-(1′,2′,4′,5′-tetraoxacyclohexane)-3′-spirocyclohexane, the structure of which was confirmed by NMR spectroscopy and an x-ray crystal structure analysis. 19β,28-Epoxy-28-oxo-18α-olean-1-en-3-one was formed as a side product during oxidative dehydration of 28-oxoallobetulone.



Similar content being viewed by others
References
G. A. Tolstikov, A. G. Tolstikov, and O. V. Tolstikova, Usp. Khim., 65 (9), 836 (1996).
V. M. Dembitsky, Eur. J. Med. Chem., 43, 223 (2008).
N. Kumar, M. Sharma, and D. S. Rawat, Curr. Med. Chem., 18, 3889 (2011).
D. L. Klayman, Science, 228, 1049 (1985).
P. M. O’Neill and G. H. Posner, J. Med. Chem., 47 (12), 2945 (2004).
R. K. Haynes, Curr. Top. Med. Chem., 6 (5), 509 (2006).
A. O. Terent’ev, A. V. Kutkin, Z. A. Starikova, M. Yu. Antipin, Yu. N. Ogibin, and G. I. Nikishin, Synthesis, 14, 2356 (2004).
C. W. Jefford, Curr. Top. Med. Chem., 12, 373 (2012).
S. A. Charman, S. Arbe-Barnes, I. C. Bathurst, R. Brun, M. Campbell, W. N. Charman, F. C. K. Chiu, J. Chollet, J. C. Craft, D. J. Creek, Y. Dong, H. Matile, M. Maurer, J. Morizzi, T. Nguyen, P. Papastogiannidis, C. Scheurer, D. M. Shackleford, K. Sriraghavan, L. Stingelin, Y. Tang, H. Urwyler, X. Wang, K. L. White, S. Wittlin, L. Zhou, and J. L. Vennerstrom, Proc. Natl. Acad. Sci. USA, 108 (11), 4400 (2011).
F. Garah, M. Wong, R. K. Amewu, S. Muangnoicharoen, J. L. Maggs, J.-L. Stigliani, B. K. Park, J. Chadwick, S. A. Ward, and P. M. O’Neill, J. Med. Chem., 54 (19), 6443 (2011).
D. V. Kazakov, A. R. Timerbaev, F. E. Safarov, T. I. Nazirov, O. B. Kazakova, G. Yu. Ismuratov, A. O. Terent’ev, D. A. Borisov, A. G. Tolstikov, G. A. Tolstikov, and W. Adam, RSC Adv., 2, 107 (2012).
D. J. Creek, W. N. Charman, F. C. K. Chiu, R. J. Prankerd, K. J. McCullough, Y. Dong, J. L. Vennerstrom, and S. A. Charman, J. Pharm. Sci., 96 (11), 2945 (2007).
Y. Dong, S. Wittlin, K. Sriraghavan, J. Chollet, S. A. Charman, W. N. Charman, C. Scheurer, H. Urwyler, T. J. Santo, C. Snyder, D. J. Creek, J. Morizzi, M. Koltun, H. Matile, X. Wang, M. Padmanilayam, Y. Tang, A. Dorn, R. Brun, and J. L. Vennerstrom, J. Med. Chem., 53, 481 (2010).
P. M. O’Neill, R. K. Amewu, G. L. Nixon, F. B. ElGarah, M. Mungthin, J. Chadwick, A. E. Shone, L. Vivas, H. Lander, V. Barton, S. Muangnoicharoen, P. G. Bray, J. Davies, B. K. Park, S. Wittlin, R. Brun, M. Preschel, K. Zhang, and S. A. Ward, Angew. Chem., Int. Ed., 49, 5693 (2010).
N. Kumar, R. Singh, and D. S. Rawat, Med. Res. Rev., 32, 581 (2012).
B. A. Solaja, N. Terzic, G. Pocsfalvi, L. Gerena, B. Tinant, D. Opsenica, and W. K. Milhous, J. Med. Chem., 45, 3331 (2002).
P. Ghorai and P. H. Dussault, Org. Lett., 11, 213 (2009).
R. Amewu, A. V. Stachulski, S. A. Ward, N. G. Berry, P. G. Bray, J. Davies, G. Labat, L. Vivasc, and P. M. O’Neill, Org. Biomol. Chem., 4, 4431 (2006).
F. Gutierrez-Nicolas, B. Gordillo-Roman, J. C. Oberti, A. Estevez-Braun, A. G. Ravelo, and P. Joseph-Nathan, J. Nat. Prod., 75, 669 (2012).
K. C. Nicolaou, T. Montagnon, P. S. Baran, and Y.-L. Zhang, J. Am. Chem. Soc., 124, 2245 (2002).
T. A. Hase, E. Soukas, and A. Weckman, Synth. Commun., 11, 489 (1981).
J. Klinot and A. Vystrcil, Collect. Czech. Chem. Commun., 31, 1079 (1966).
W.-H. Hui and M.-M. Li, Phytochemistry, 15, 561 (1976).
D. M. Doddrell, D. T. Pegg, and M. R. Bendall, J. Magn. Reson., 48, 323 (1982).
K. Nagayama, A. Kumar, K. Wuthrich, and R. R. Ernst, J. Magn. Reson., 40, 321 (1980).
A. L. Davis, J. Keeler, E. D. Laue, and D. Moskau, J. Magn. Reson., 98, 207 (1992).
W. Willker, D. Leibfritz, R. Kerssebaum, and W. Bermel, Magn. Reson. Chem., 31, 287 (1993).
R. Wagner and S. Berger, J. Magn. Reson. A, 123, 119 (1996).
O. B. Flekhter, L. R. Nigmatullina, L. A. Baltina, L. T. Karachurina, F. Z. Galin, F. S. Zarudii, G. A. Tolstikov, E. I. Boreko, N. I. Pavlova, S. N. Nikolaeva, and O. V. Savinova, Khim.-farm. Zh., 36 (9), 26 (2002).
K. Zymitek, M. Zupan, S. Stavber, and J. Iskra, Org. Lett., 8, 2491 (2006).
APEX2 and SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2005.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 64, 112 (2008).
Acknowledgment
The work was sponsored by the RFBR (Projects Nos. 09-03-00831 and 14-03-31664) and a grant of the Russian Federation President for State Support of Young Russian Scientists (MD-3852.2009.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2015, pp. 87–91.
Rights and permissions
About this article
Cite this article
Yamansarov, E.Y., Kazakova, O.B., Lobov, A.N. et al. Synthesis of a Triterpenoid with a 1,2,4,5-Tetraoxane Fragment. Chem Nat Compd 51, 97–102 (2015). https://doi.org/10.1007/s10600-015-1211-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1211-1