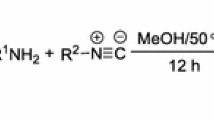Alternative and complementary procedures were adopted for preparing 2-arylazetidine derivatives in moderate to good yields. Preliminary biological evaluation of 2-arylazetidines as ligands of nicotinic acetylcholine receptors allowed to identify chloro-substituted analogs as the most interesting congeners. The title compounds may be considered as suitable hit compounds for developing new nicotinic acetylcholine receptor ligands that may be safer than the currently available drugs targeting nicotinic acetylcholine receptors. Our described synthetic approaches enable facile access to a large number of diversely decorated azetidines for studying the structure–activity relationships and for refining the toxico-pharmacological profile of these agents.

Similar content being viewed by others
References
Forgacs, P. B.; Bodis-Wollner, I. J. Neural Transm. 2004, 111, 1317.
(a) Hurst, R.; Rollema, H.; Bertrand, D. Pharmacol. Ther. 2013, 137, 22. (b) Dineley, K. T.; Pandya, A. A.; Yakel, J. L. Trends Pharmacol. Sci. 2015, 36, 96.
(a) Decker, M. W.; Meyer, M. D.; Sullivan, J. P. Expert Opin. Invest. Drugs 2001, 10, 1819. (b) Zhang, X.; Xiao, H. S. Curr. Opin. Mol. Ther. 2005, 7, 532. (c) Vincler, M. Expert Opin. Invest. Drugs 2005, 14, 1191. (d) Nirogi, R.; Goura, V; Abraham, R.; Jayarajan, P. Eur. J. Pharmacol. 2013, 712, 22.
Picciotto, M. R.; Lewis, A. S.; van Schalkwyk, G. I.; Mineur, Y. S. Neuropharmacology 2015, 96, 235.
Dwoskin, L. P.; Smith, A. M.; Wooters, T. E.; Zhang, Z.; Crooks, P. A.; Bardo, M. T. Biochem. Pharmacol. 2009, 78, 732.
Sacco, K. A.; Bannon, K. L.; George, T. P. J. Psychopharmacology 2004, 18, 457.
Quik, M.; Zhang, D.; Perez, X. A.; Bordia, T. Pharmacol. Ther. 2014, 144, 50.
(a) Kumari, V.; Postma, P. Neurosci. Biobehav. Rev. 2005, 29, 1021. (b) de Leon, J.; Diaz, F. J.; Aguilar, M. C.; Jurado, D.; Gurpegui, M. Schizophr. Res. 2006, 86, 256.
Lloyd, G. K.; Williams, M. J. Pharmacol. Exp. Ther. 2000, 292, 461.
McEvoy, J. P.; Allen, T. B. Curr. Drug Targets: CNS Neurol. Disord. 2002, 1, 433.
Czodrowski, P.; Mallinger, A.; Wienke, D.; Esdar, C.; Pöschke, O.; Busch, M.; Rohdich, F.; Eccles, S. A.; Ortiz-Ruiz, M.-J.; Schneider, R.; Raynaud, F. I.; Clarke, P. A.; Musil, D.; Schwarz, D.; Dale, T.; Urbahns, K.; Blagg, J.; Schiemann, K. J. Med. Chem. 2016, 59, 9337.
Jacobson, T. A.; Ito, M. K.; Maki, K. C.; Orringer, C. E.; Bays, H. E.; Jones, P. H.; McKenney, J. M.; Grundy, S. M.; Gill, E. A.; Wild, R. A.; Wilson, D. P.; Brown, W. V. J. Clin. Lipidol. 2015, 9, 129.
López-Muñoz, F.; Alamo, C. Curr. Pharm. Des. 2009, 15, 1563.
Changeux, J. P.; Edelstein, S. J. Neuron 1998, 21, 959.
(a) Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988. (b) Han, J.-Q.; Zhang, H.-H.; Xu, P.-F.; Luo, Y.-C. Org. Lett. 2016, 18, 5212. (c) Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai, H. Org. Lett. 2013, 15, 4142. (d) Das, B.; Balasubramanyam, P.; Veeranjaneyulu, B.; Reddy, G. C. J. Org. Chem. 2009, 74, 9505.
(a) Degennaro, L.; Zenzola, M.; Trinchera, P.; Carroccia, L.; Giovine, A.; Romanazzi, G.; Falcicchio, A.; Luisi, R. Chem. Commun. 2014, 50, 1698. (b) Zenzola, M.; Degennaro, L.; Trinchera, P.; Carroccia, L.; Giovine, A.; Romanazzi, G.; Mastrorilli, P.; Rizzi, R.; Pisano, L.; Luisi, R. Chem.–Eur. J. 2014, 20, 12190. (c) De Angelis, S.; De Renzo, M.; Carlucci, C.; Degennaro, L.; Luisi R. Org. Biomol. Chem. 2016, 14, 4304.
(a) Parisi, G.; Zenzola, M.; Capitanelli, E.; Carlucci, C.; Romanazzi, G.; Pisano, L.; Degennaro, L.; Luisi, R. Pure Appl. Chem. 2016, 88, 631. (b) Parisi, G.; Capitanelli, E.; Pierro, A.; Romanazzi, G.; Clarkson, G. J.; Degennaro, L.; Luisi, R. Chem. Commun. 2015, 51, 15588. (c) De Ceglie, M. C.; Musio, B.; Affortunato, F.; Moliterni, A.; Altomare, A.; Florio, S.; Luisi, R. Chem.–Eur. J. 2011, 17, 286.
(a) Hopkins, A. L.; Keserü, G. M.; Leeson, P. D.; Rees, D. C.; Reynolds, C. H. Nat. Rev. Drug Discovery 2014, 13, 105. (b) Gualdani, R.; Cavalluzzi, M. M.; Lentini, G. Curr. Med. Chem. 2016, 23, 2289. (c) Gualdani, R.; Cavalluzzi, M. M.; Lentini, G.; Habtemariam, S. Molecules 2016, 21, 1530.
Hogg, R. C.; Bertrand, D. Biochem. Pharmacol. 2007, 73, 459.
Carocci, A.; Lentini, G.; Catalano, A.; Cavalluzzi, M. M.; Bruno, C.; Muraglia, M.; Colabufo, N. A.; Galeotti, N.; Corbo, F.; Matucci, R.; Ghelardini, C.; Franchini, C. ChemMedChem 2010, 5, 696.
Lo, M. M.-C., Fu, G. C. Tetrahedron 2001, 57, 2621.
Roselli, M.; Lentini, G.; Habtemariam, S. Phytother. Res. 2012, 26, 908.
Manni, M. E.; Bigagli, E.; Lodovici, M.; Zazzeri, M.; Raimondi, L. Pharmacol. Res. 2012, 65, 465.
We thank the University of Bari for support. We are grateful to Dr. Laura Carroccia for precious contribution to the work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(3), 329–334
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1445 kb)
Rights and permissions
About this article
Cite this article
Degennaro, L., Zenzola, M., Laurino, A. et al. 2-Arylazetidines as ligands for nicotinic acetylcholine receptors. Chem Heterocycl Comp 53, 329–334 (2017). https://doi.org/10.1007/s10593-017-2061-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2061-5